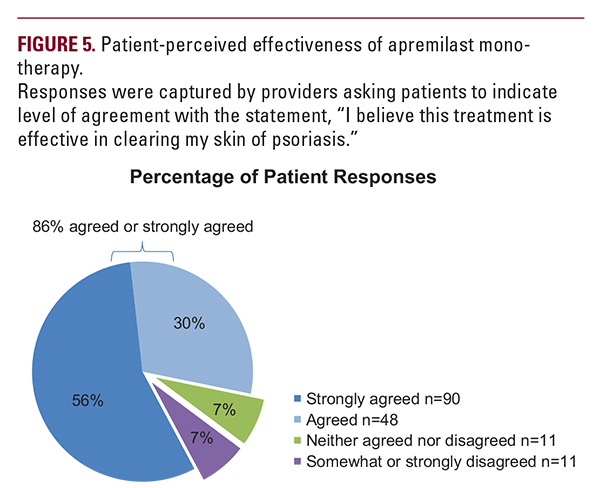
 systemic-experienced patients had mean PGA scores of 2.79 and 2.48 and mean psoriasis-affected BSA of 17.85% and 12.93%, respectively. Their baseline demographic characteristics and pattern of comorbidities were also reflective of the broader population of US patients with moderate to severe psoriasis, with >50% having psoriasis-related comorbidities.1,12-14 Most patients who switched to apremilast monotherapy were previously receiving only topical therapy, indicating inadequate treatment and a possible place for apremilast in the treatment of patients with psoriasis immediately after topical therapy is no longer adequately managing symptoms. The current ndings from dermatology clinical practices confirm those from the phase 3 ESTEEM 1 and 2 clinical trials, which demonstrated efficacy of apremilast in patients with moderate to severe plaque psoriasis.9,10 Approximately one-quarter of patients achieved low disease severity as measured by PGA of 0 or 1 after approximately 2 months of apremilast therapy, which is consistent with reductions in PGA observed in apremilast clinical trials.9,10 In our study, PGA and BSA scores were each significantly reduced by approximately 60% with apremilast monotherapy within 6 months of treatment initiation, regardless of whether patients were systemic-naive or systemic-experienced. Patients who were systemic-naive had slightly better efficacy responses, indicating that these patients might be a more appropriate population for apremilast than systemic-experienced patients. Clinically significant reductions in itch were seen in both systemic-naive and systemic-experienced patients.15 Although the decrease in itch NRS did not reach statistical significance in systemic-naive patients, systemic-experienced patients reported a significant reduction within 6 months after apremilast initiation, approaching a score of 0 approximately 5 months after apremilast initiation and complete resolution in some patients.The majority of patients (86.2%) considered apremilast to be effective in clearing their skin of psoriasis, suggesting a high level of patient satisfaction with treatment. Both treatment experience groups had reductions in body weight that were comparable to those observed in clinical trials of apremilast.9,10
systemic-experienced patients had mean PGA scores of 2.79 and 2.48 and mean psoriasis-affected BSA of 17.85% and 12.93%, respectively. Their baseline demographic characteristics and pattern of comorbidities were also reflective of the broader population of US patients with moderate to severe psoriasis, with >50% having psoriasis-related comorbidities.1,12-14 Most patients who switched to apremilast monotherapy were previously receiving only topical therapy, indicating inadequate treatment and a possible place for apremilast in the treatment of patients with psoriasis immediately after topical therapy is no longer adequately managing symptoms. The current ndings from dermatology clinical practices confirm those from the phase 3 ESTEEM 1 and 2 clinical trials, which demonstrated efficacy of apremilast in patients with moderate to severe plaque psoriasis.9,10 Approximately one-quarter of patients achieved low disease severity as measured by PGA of 0 or 1 after approximately 2 months of apremilast therapy, which is consistent with reductions in PGA observed in apremilast clinical trials.9,10 In our study, PGA and BSA scores were each significantly reduced by approximately 60% with apremilast monotherapy within 6 months of treatment initiation, regardless of whether patients were systemic-naive or systemic-experienced. Patients who were systemic-naive had slightly better efficacy responses, indicating that these patients might be a more appropriate population for apremilast than systemic-experienced patients. Clinically significant reductions in itch were seen in both systemic-naive and systemic-experienced patients.15 Although the decrease in itch NRS did not reach statistical significance in systemic-naive patients, systemic-experienced patients reported a significant reduction within 6 months after apremilast initiation, approaching a score of 0 approximately 5 months after apremilast initiation and complete resolution in some patients.The majority of patients (86.2%) considered apremilast to be effective in clearing their skin of psoriasis, suggesting a high level of patient satisfaction with treatment. Both treatment experience groups had reductions in body weight that were comparable to those observed in clinical trials of apremilast.9,10 Limitations
Data represent dermatology providers’ EMR entries, and reporting may not be uniform across providers. Medication data reflect what dermatology providers prescribed; medication adherence was not evaluated. The statistical power of efficacy subanalyses may be limited by small numbers of patients with evaluable data.
CONCLUSION
Based on this retrospective, multicenter, longitudinal, observational cohort study using an EMR database, apremilast was prescribed to patients with features typical of a population with moderate to severe psoriasis, including a high prevalence of comorbid conditions. With up to 6 months of apremilast mono-therapy, psoriasis severity was reduced as measured by PGA and BSA, regardless of whether patients were systemic-naive or systemic-experienced, with slightly better responses seen in the systemic-naive patient population. Most patients considered apremilast to be effective in reducing their psoriasis symptoms.
DISCLOSURES
April Armstrong: AbbVie, Janssen, Lilly, Modernizing Medicine, Novartis, Pfizer, Regeneron, and Sanofi – grants, consulting fees, and/or honorarium. Eugenia Levi: Celgene Corporation – employment.
ACKNOWLEDGMENTS
The authors received editorial support in the preparation of this report from Amy Shaberman, PhD, of Peloton Advantage, LLC, Parsippany, NJ, USA, funded by Celgene Corporation, Summit, NJ, USA. The authors, however, directed and are fully responsible for all content and editorial decisions for this manuscript.
REFERENCES
- Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47(1):37-45.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512-516.
- Parisi R, Symmons DP, Grif ths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377-385.
- Dubertret L, Mrowietz U, Ranki A, et al. European patient perspectives on the impact of psoriasis: the EUROPSO patient membership survey. Br J Dermatol. 2006;155(4):729-736.
- Armstrong AW, Robertson AD, Wu J, et al. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003-2011. JAMA Dermatol. 2013;149(10):1180-1185.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70(5):871-881.
- van de Kerkhof PCM, Reich K, Kavanaugh A, et al. Physician perspectives in the managment of psoriasis and psoriatic arthritis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Eur Acad Dermatol Venereol. 2015;29(10):2002-2010.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. 2011;65(1):137-174.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM 1]). J Am Acad Dermatol. 2015;73(1):37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173(6):1387-1399.
- Ohtsuki M, Okubo Y, Komine M, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of Japanese patients with moderate to severe plaque psoriasis: Efficacy, safety and tolerability results from a phase 2b randomized controlled trial. J Dermatol. 2017;44(8):873-884.
- Yeung H, Wan J, Van Voorhees AS, et al. Patient-reported reasons for the discontinuation of commonly used treatments for moderate to severe pso- riasis. J Am Acad Dermatol. 2013;68(1):64-72.
- Davidovici BB, Sattar N, Prinz J, et al. Psoriasis and systemic in ammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130(7):1785-1796.
- Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: results from the National Psoriasis Foundation surveys 2003 to 2011. Dermatology. 2012;225(2):121-126.
- Sobell JM, Foley P, Toth D, et al. Effects of apremilast on pruritus and skin dis- comfort/pain correlate with improvements in quality of life in patients with moderate to severe plaque psoriasis. Acta Derm Venereol. 2016;96(4):514- 520.
AUTHOR CORRESPONDENCE
April Armstrong MD MPH april.armstrong@med.usc.edu






