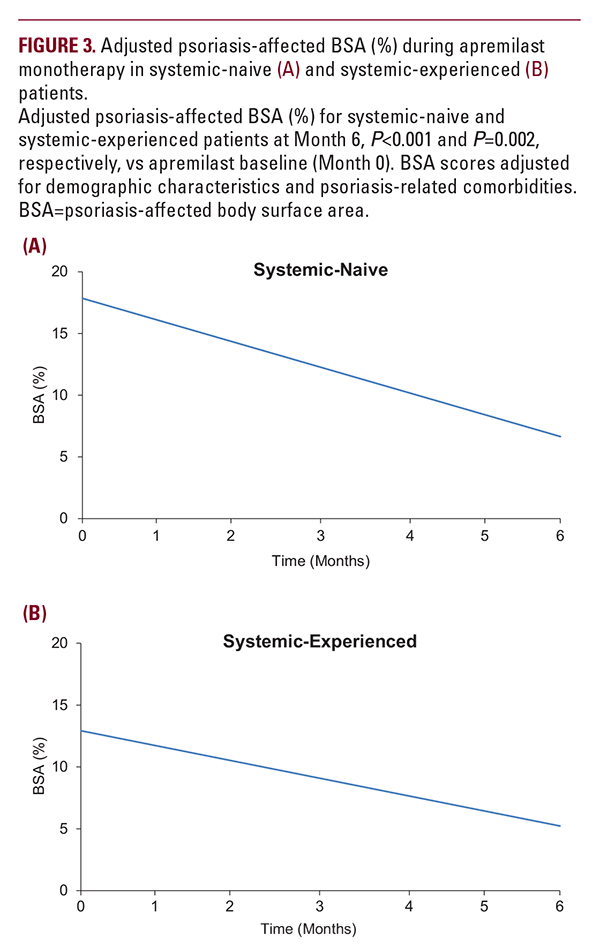
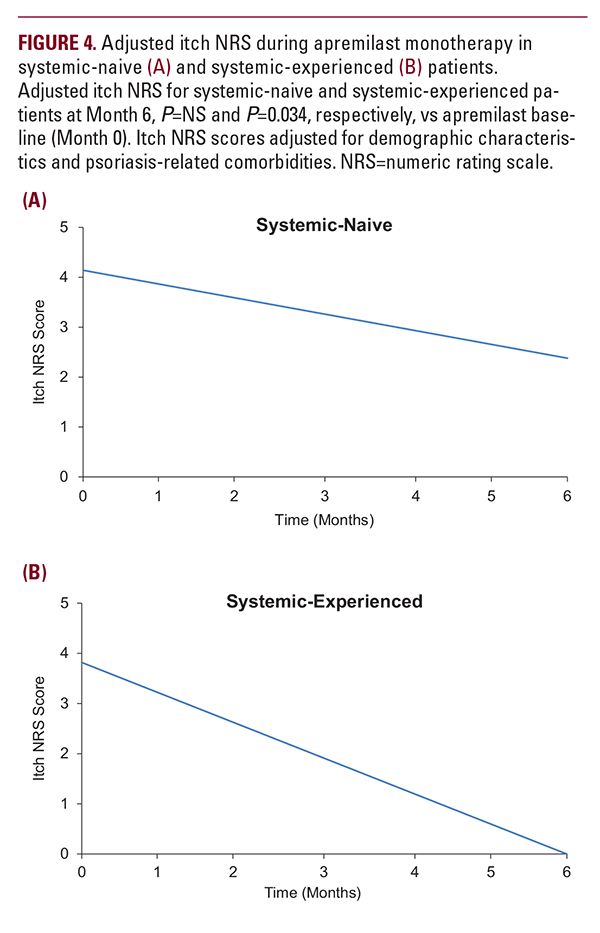
 did not reach statistical signi cance. In systemic-experienced patients, adjusted itch NRS scores decreased by 3.82 points (P=0.034), approaching a score of 0 approximately 5 months after apremilast initiation (Figure 4).
did not reach statistical signi cance. In systemic-experienced patients, adjusted itch NRS scores decreased by 3.82 points (P=0.034), approaching a score of 0 approximately 5 months after apremilast initiation (Figure 4). Changes in Body Weight
A total of 352 patients (n=179 systemic-naive; n=173 systemic-experienced) had body weight recorded at ≥2 visits during the study period. At apremilast initiation, the mean adjusted body weight was 76.45 kg in systemic-naive patients and 75.66 kg in systemic-experienced patients. Mean decrease from baseline in body weight was −1.75 kg in systemic-naive patients and −1.09 kg in systemic-experienced patients.
POTE of Apremilast Monotherapy
Among patients who received apremilast monotherapy for ≥90 days and had ≥1 POTE assessment (n=160), the majority (n=138; 86.2%) agreed or strongly agreed that apremilast treatment was effective in clearing their skin of psoriasis (Figure 5).
DISCUSSION
Apremilast has demonstrated efficacy in the treatment of adult patients with moderate to severe plaque psoriasis in phase 2 and 3 clinical trials.9-11 Clinical trial data, however, do not fully reflect the demographically and clinically diverse population of patients treated at dermatology practices. Determining treatment patterns and clinical effectiveness of therapies at the point of care is critical for ascertaining real-world patient out- comes, devising treatment strategies, and improving patient outcomes. EMR databases can serve as an important source of real-world data for outcomes research. Based on data captured from >5000 US dermatology providers, apremilast-treated patients appeared to have chronic plaque psoriasis that was at least moderate in severity. Specifically, at the time of apremilast initiation, systemic-naive and






