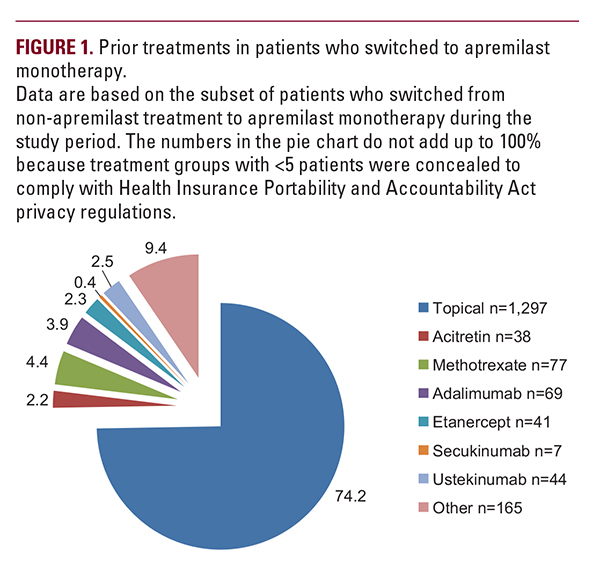
 almost three-quarters (74.2%) changed from topical treatment alone to apremilast monotherapy (Figure 1). Most patients who started on phototherapy, methotrexate, adalimumab, or ustekinumab received apremilast as add-on therapy to their ongoing treatment (range, 78.1% to 88.1%).
almost three-quarters (74.2%) changed from topical treatment alone to apremilast monotherapy (Figure 1). Most patients who started on phototherapy, methotrexate, adalimumab, or ustekinumab received apremilast as add-on therapy to their ongoing treatment (range, 78.1% to 88.1%). Effect of Apremilast Monotherapy on PGA
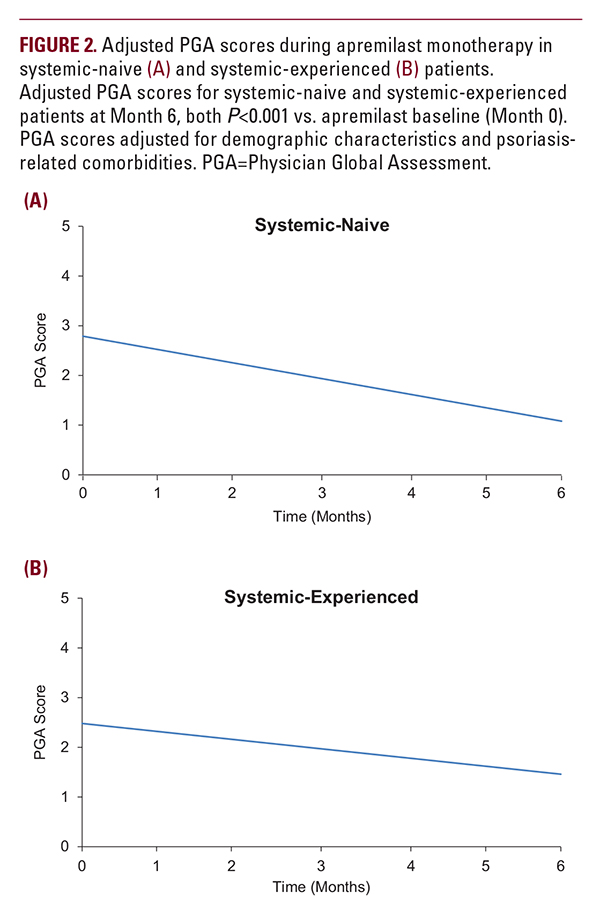
A total of 381 patients (n=173 systemic-naive; n=208 systemic-experienced) on apremilast monotherapy met inclusion criteria for analysis of PGA scores (ie, they had PGA values recorded at ≥2 visits during the study period). In this patient subgroup, mean adjusted PGA scores at the time of apremilast initiation were 2.79 and 2.48 for systemic-naive and systemic-experienced patients, respectively. PGA scores de- creased significantly in both the systemic-naive patients (−1.71; P less than 0.001; approximately −61%) and the systemic-experienced patients (−1.02; P less than 0.001; approximately −41%) within 6 months of initiating apremilast monotherapy (Figure 2). A total of 360 patients met inclusion criteria for analysis of PGA score achievement of 0 or 1 (ie, patients with PGA values recorded at ≥2 visits during the study period, and with ≥1 PGA score ≥2; n=168 systemic-naive; n=192 systemic-experienced). Among these, 45 (26.8%) systemic-naive and 49 (25.5%) systemic-experienced patients had a PGA score of 0 or 1 at fol- low-up within 6 months of treatment initiation.Themediantime to achievement of low disease severity was 62 and 63 days for systemic-naive and systemic-experienced patients, respectively.
Effect of Apremilast Monotherapy on BSA
A total of 373 patients (n=196 systemic-naive; n=177 systemic-experienced) on apremilast monotherapy met inclusion criteria for analysis of BSA scores (ie, BSA values recorded at ≥2 visits during the study period). In this patient subgroup, at apremilast initiation, mean adjusted BSA was 17.85% and 12.93% for systemic-naive and systemic-experienced patients, respectively, and significantly decreased by 11.12 and 7.70 percentage points (P less than 0.001) within 6 months of initiating apremilast monotherapy (Figure 3). These BSA reductions represent a decrease from baseline of approximately 62% in systemic-naive patients and a decrease from baseline of ap- proximately 60% in systemic-experienced patients.
Effect of Apremilast Monotherapy on Itch NRS
A total of 51 patients (n=28 systemic-naive; n=23 systemic-experienced) had itch NRS values recorded at ≥2 visits during the study period. At apremilast initiation, mean adjust- ed itch NRS scores were 4.14 and 3.82 for systemic-naive and systemic-experienced patients, respectively. Within 6 months of apremilast initiation, adjusted itch NRS scores decreased by 1.76 points in systemic-naive patients, although the decrease






