offices at the point of care. To address this knowledge gap, this study examined prescribing patterns, clinical effectiveness, and patient-perceived overall treatment effectiveness (POTE) of apremilast in the US dermatology practice setting using Modernizing Medicine’s electronic medical record (EMR) database of >5000 US dermatology providers and >550,000 psoriasis patients.
METHODS
Study Design
This was a retrospective, multicenter, longitudinal, observational cohort study that examined outcomes from adults with psoriasis in real-world dermatology practices in 49 states and 2 territories across the United States. The study period was from October 1, 2014, through January 31, 2016. Structured data were collected from Modernizing Medicine’s EMR database Electronic Medical Assistant® (EMATM), which is a dermatology-specific, Health Insurance Portability and Accountability Act-compliant EMR platform, from >5000 dermatology practices. During clinic visits, data were entered directly into the EMR system by dermatology providers and their staff at the point of care.
Patient Inclusion Criteria
Patients eligible for study inclusion were adults ≥18 years of age with a dermatologist-given, psoriasis-specific diagnosis (ICD-9, ICD-10) who at study initiation or at any time during the study period received apremilast either alone or in combination with other psoriasis treatments. Psoriasis was defined as any of the following psoriasis-specific diagnoses, which were selected by a dermatologist in the EMR database during routine clinical encounters: “psoriasis,” “psoriasis vulgaris,” “generalized plaque psoriasis,” “localized plaque psoriasis,” “localized scalp psoriasis,” “palmoplantar psoriasis,” “nail psoriasis,” “guttate psoriasis,” “inverse psoriasis,” and “ostraceous psoriasis.”
Assessments and Analysis
Patient demographic and disease characteristics were recorded at the most recent clinic visit. Frequencies of psoriasis-related comorbidities were determined. Efficacy and weight outcomes were evaluated for patients who received apremilast mono- therapy and who had ≥2 efficacy assessments of the same outcome during the study period, the first at the time of initial apremilast prescription and ≥1 thereafter within 6 months. For analyses of apremilast monotherapy, patients were stratified according to whether they had received any conventional or biologic systemic treatment prior to initiation of apremilast (naive/experienced). Outcomes at Month 6 that were compared with apremilast baseline included Physician Global Assessment of Disease Severity (PGA; 0=clear, 1=minimal, 2=mild, 3=moderate, 4=marked, and 5=severe); achievement of low disease severity (PGA score of 0 or 1) in patients with ≥1 PGA score ≥2; percentage of psoriasis-involved body surface area (BSA; 0% to 100%); itch numeric rating scale (NRS; 0 to 10; 0=no itch, 10=worst itch possible); and changes in body weight. Longitudinal changes in PGA, BSA, itch NRS, and body weight were examined using a linear mixed-effect model, adjusted for demographic characteristics and psoriasis-related comorbidities. POTE was examined among patients receiving apremilast monotherapy for ≥90 days using a 5-point Likert scale (1=strongly agree, 5=strongly disagree) in response to the following statement: “I believe this treatment is effective in clearing my skin of psoriasis.” The POTE assessment from the most recent clinic visit was included in the analysis. Frequency of each response category was determined.
RESULTS
Patients
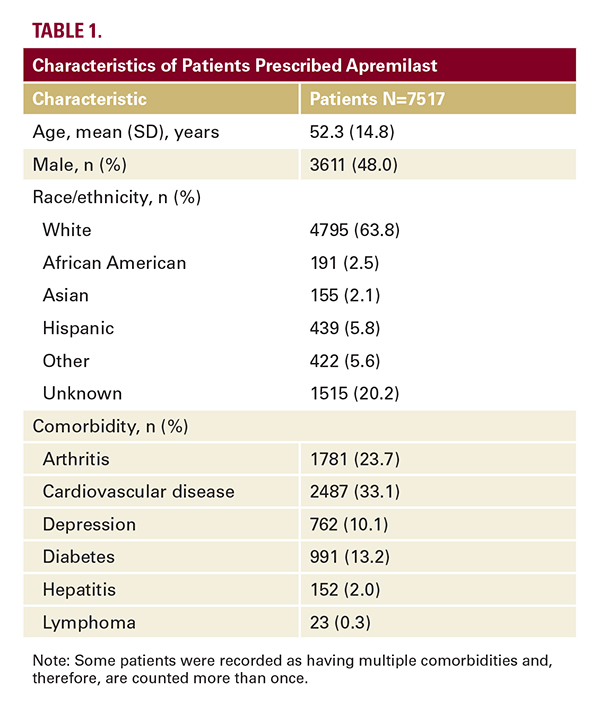
A total of 7517 patients received apremilast during the study period and were included in the analysis of apremilast prescribing patterns. Demographic characteristics are summarized in the Table. More than one-half of the patients (52.4%) had a psoriasis-related comorbidity. The most common comorbidity was cardiovascular disease (33.1% of patients).
Treatment Patterns With Apremilast
Among the patients who changed from non-apremilast treatments to apremilast monotherapy during the study period,