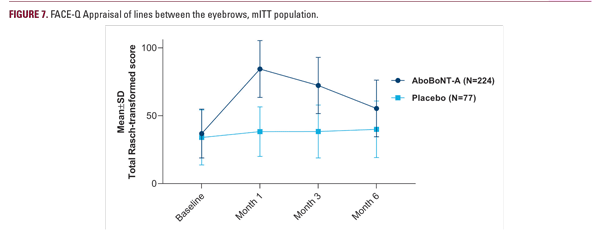
Subjects tended to perceive themselves as younger after treatment with aboBoNT-A. The largest improvement was at month 1, when subjects reported looking 2.1 years younger than before aboBoNT-A treatment (mean value), while the placebo group increased their perceived age by a mean of 0.4 year at month 1 after treatment.
Safety
An overview of TEAEs is presented in Table 2. Serious events occurred in 4 subjects (1.8%) in the aboBoNT-A group and in no subjects in the placebo group. None of these events were related to treatment.
Treatment-related TEAEs occurred in 10.8% of subjects in the aboBoNT-A group and 7.8% in the placebo group, all were of mild or moderate intensity. The most common treatmentrelated TEAEs during the study, occurring in >1 subject in total, were headache, eyelid ptosis and injection-site pain (Table 2). Three subjects (1.3%) developed eyelid ptosis after injection of aboBoNT-A. All ptosis events were mild in intensity and resolved without intervention. No new safety signals were identified.

DISCUSSION
The aim of this study was to evaluate GL treatment using a new higher dilution of the 300-U-vial of aboBoNT-A powder, and a volume of injection of 0.1 mL. The safety results for this injection volume were consistent with the well-known safety profile of aboBoNT-A and in-line with the current prescribing information.1,7-9 No new safety signals were identified. Treatment with aboBoNT-A was well tolerated and there was a low rate of injection site pain (0.4% in the aboBoNT-A group). All treatmentrelated TEAEs were non-serious and mild or moderate in intensity. Eyelid ptosis, all of mild intensity, was reported in 1.3% of subjects, which is lower than in the prescribing information for aboBoNT-A,1 and for other toxin products used in glabellar line treatment including onabotulinumtoxinA10 and prabotulinumtoxinA-xvfs,11 indicating no increase in local spread of toxin with the larger injection volume of aboBoNT-A.
The present study confirmed efficacy also for this dilution and injection volume, including a rapid onset of effect, with a median time to onset of 2 days reported in the subject diary, and a ≥1-grade improvement in GL severity on day 2 reported for 74% and 65% of subjects in the investigator- and subjectassessments, respectively. This is similar to the onset time for aboBoNT-A demonstrated in prior studies.3,8,12-14
In line with previously reported data on aboBoNT-A,7,13 high responder rates were attained on both GL severity scales at 2–4 weeks after treatment with the 0.1-mL-injection volume, including a none-or-mild response in 92% of subjects and a ≥1-grade response in 95%–100% of subjects. The GL severity improvement was sustained up to 6 months after aboBoNT-A treatment, with statistically significant higher responder rates over placebo both in the investigator and subject assessments. Approximately half of the subjects (46% based on ILA; 56%
The present study confirmed efficacy also for this dilution and injection volume, including a rapid onset of effect, with a median time to onset of 2 days reported in the subject diary, and a ≥1-grade improvement in GL severity on day 2 reported for 74% and 65% of subjects in the investigator- and subjectassessments, respectively. This is similar to the onset time for aboBoNT-A demonstrated in prior studies.3,8,12-14
In line with previously reported data on aboBoNT-A,7,13 high responder rates were attained on both GL severity scales at 2–4 weeks after treatment with the 0.1-mL-injection volume, including a none-or-mild response in 92% of subjects and a ≥1-grade response in 95%–100% of subjects. The GL severity improvement was sustained up to 6 months after aboBoNT-A treatment, with statistically significant higher responder rates over placebo both in the investigator and subject assessments. Approximately half of the subjects (46% based on ILA; 56%






