The study population consisted of 88% women and 92% White subjects. Randomized subjects were aged between 21 and 66 years, mean, 44.1 years, and 52% were toxin naive. More details are provided in Table 1.
Efficacy
Time to Onset of Response
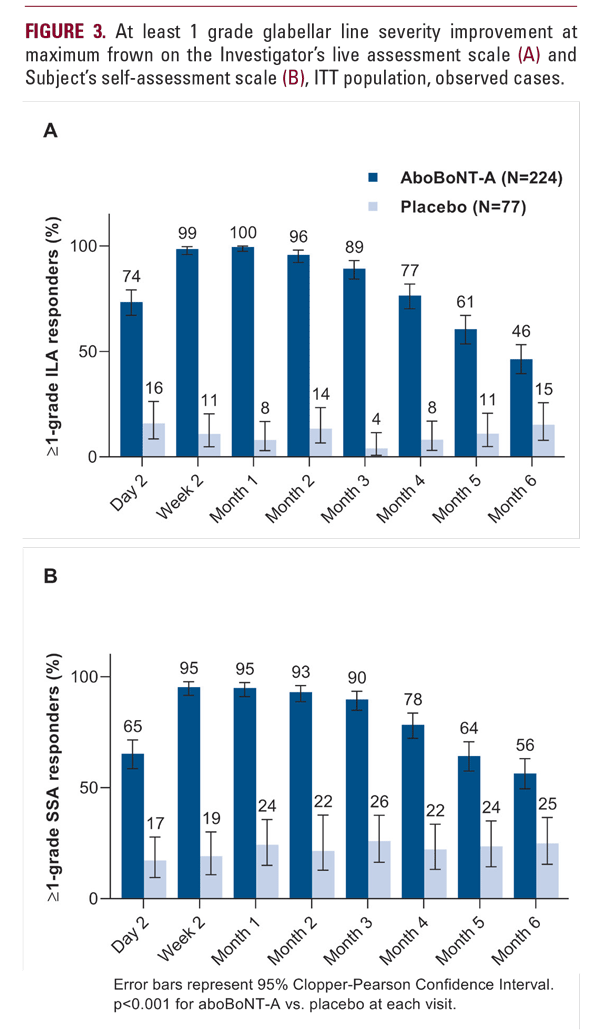
The median subject-reported time to onset of effect was 2.0 days after aboBoNT-A treatment based on the subject diary, with 34% reporting onset on day 1 and 60% by day 2 (Figure 1). For placebo, no median time to onset could be calculated due to few subjects reporting an effect (11 of 77).
The investigator assessments of GL severity (ILA) at day 2 showed 47% of subjects with none or mild GL severity and


Efficacy
Time to Onset of Response
The median subject-reported time to onset of effect was 2.0 days after aboBoNT-A treatment based on the subject diary, with 34% reporting onset on day 1 and 60% by day 2 (Figure 1). For placebo, no median time to onset could be calculated due to few subjects reporting an effect (11 of 77).
The investigator assessments of GL severity (ILA) at day 2 showed 47% of subjects with none or mild GL severity and









