METHODS
Study Subjects
Eligible subjects were healthy women 21 to 65 years old with Fitzpatrick skin types I to IV and self-perceived thinning hair, as confirmed by the investigator. Subjects agreed to follow study procedures, provide a negative pregnancy test, and use a sound method of contraception (if within childbearing years), as well as maintain their current diet, medications, exercise routines, hair shampooing, and color treatment frequency for the duration of the study. The study was approved by an institutional review board and conducted in compliance with good clinical practice. All participants provided written informed consents prior to participating.Reasons for exclusion from study participation included: participation in other clinical research; pregnancy or nursing; recent
Test Material
Subjects were randomized in a double-blinded fashion 2:1 to receive a novel oral nutraceutical supplement (Nutrafol Women’s Capsules; Nutraceutical Wellness, Inc., New York, NY) or placebo. The investigational product contains a patent-pending Synergen Complex, a proprietary blend of standardized clinically-tested and bio-optimized phytoactive extracts, and additional vitamins, minerals, and botanicals. Some of the key ingredients in the supplement include standardized extracts of ashwagandha, curcumin, saw palmetto, tocotrienol-rich tocotrienol/tocopherol complex, piperine, and capsaicin, as well as hydrolyzed marine collagen, hyaluronic acid, and organic kelp. Placebo treatment consisted of inert capsules with same appearance. Subjects were instructed to take 4 capsules of their assigned treatment once daily with a meal or immediately following a meal at approximately the same time each day.
Study Procedures
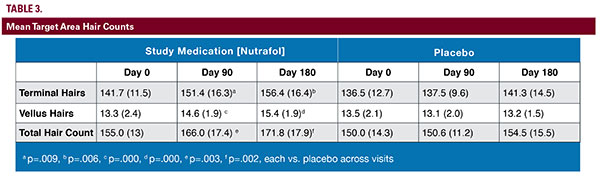
The study consisted of 3 clinic visits at baseline, day 90, and day 180. The investigator performed a physical examination at each visit, which included a basic body systems overview, vital signs, and scalp examination to rule out possible confounding scalp conditions. A urine pregnancy test was obtained from women of childbearing years. Subjects were also queried on general lifestyle practices, including exercise, smoking, alcohol, diet, and stress.During the baseline visit, a 1 cm2 target area was selected on the anterior lateral triangle of the scalp along the frontalis bone where the frontal and lateral hairlines meet. This area was recorded for further assessments using a 3-point location based on measurements taken from the medial canthus, lateral canthus, and preauricular skin pit to the hairline junction. The target area was marked with the center indicating the 3-point triangulation point.

Eligible subjects were healthy women 21 to 65 years old with Fitzpatrick skin types I to IV and self-perceived thinning hair, as confirmed by the investigator. Subjects agreed to follow study procedures, provide a negative pregnancy test, and use a sound method of contraception (if within childbearing years), as well as maintain their current diet, medications, exercise routines, hair shampooing, and color treatment frequency for the duration of the study. The study was approved by an institutional review board and conducted in compliance with good clinical practice. All participants provided written informed consents prior to participating.Reasons for exclusion from study participation included: participation in other clinical research; pregnancy or nursing; recent
Test Material
Subjects were randomized in a double-blinded fashion 2:1 to receive a novel oral nutraceutical supplement (Nutrafol Women’s Capsules; Nutraceutical Wellness, Inc., New York, NY) or placebo. The investigational product contains a patent-pending Synergen Complex, a proprietary blend of standardized clinically-tested and bio-optimized phytoactive extracts, and additional vitamins, minerals, and botanicals. Some of the key ingredients in the supplement include standardized extracts of ashwagandha, curcumin, saw palmetto, tocotrienol-rich tocotrienol/tocopherol complex, piperine, and capsaicin, as well as hydrolyzed marine collagen, hyaluronic acid, and organic kelp. Placebo treatment consisted of inert capsules with same appearance. Subjects were instructed to take 4 capsules of their assigned treatment once daily with a meal or immediately following a meal at approximately the same time each day.
Study Procedures
The study consisted of 3 clinic visits at baseline, day 90, and day 180. The investigator performed a physical examination at each visit, which included a basic body systems overview, vital signs, and scalp examination to rule out possible confounding scalp conditions. A urine pregnancy test was obtained from women of childbearing years. Subjects were also queried on general lifestyle practices, including exercise, smoking, alcohol, diet, and stress.During the baseline visit, a 1 cm2 target area was selected on the anterior lateral triangle of the scalp along the frontalis bone where the frontal and lateral hairlines meet. This area was recorded for further assessments using a 3-point location based on measurements taken from the medial canthus, lateral canthus, and preauricular skin pit to the hairline junction. The target area was marked with the center indicating the 3-point triangulation point.