
Phototrichograms were taken of the target area at each visit using macrophotographs (Canon PowerShot® G16 digital camera and 3GEN Dermlite® FOTO Pro dermoscopy lens). A 2cm2 marking template placed around the triangulation point and target area was used for creating the bundle of hair to be measured for hair mass index (HMI) (HairCheck™, Divi International Co.) at each visit. Ten terminal hairs were randomly selected at the boarder of the area and cut at the base of the scalp, and the mean diameter of the hairs was measured at each visit using microscopic digital images (Dino-Lite Digital Microscope; AnMo Electronics Corporation). At each study visit, 2-D standardized global photographs (6 views) were obtained of the entire head, scalp, and target area using standardized lighting and set-up (IntelliStudio® System; Canfield Scientific, Inc.) All 2-D images were used to assist in grading overall general hair growth, hair quality, coverage, and fullness assessments.At visits 2 and 3, with the assistance of global photographs, the blinded investigator assessed Global Hair Growth and Global Hair Quality Improvement (hair brittleness, dryness, texture, shine, scalp coverage, and overall appearance) compared with baseline. Scoring was based on a 7-point scale where -3=greatly decreased/worsened, -2=moderately decreased/worsened, -1=slightly decreased/worsened, 0=no change, +1=slightly increased/improved, +2=moderately increased/improved, and +3=significantly increased/improved.Subjects completed the Women’s Hair Loss Quality of Life (QOL)22 on all visits, and the Self-Assessment questionnaire (SAQ) (Table 1) and Ease of Use questionnaire (Table 2) at day 90 and day 180.
Study Endpoints
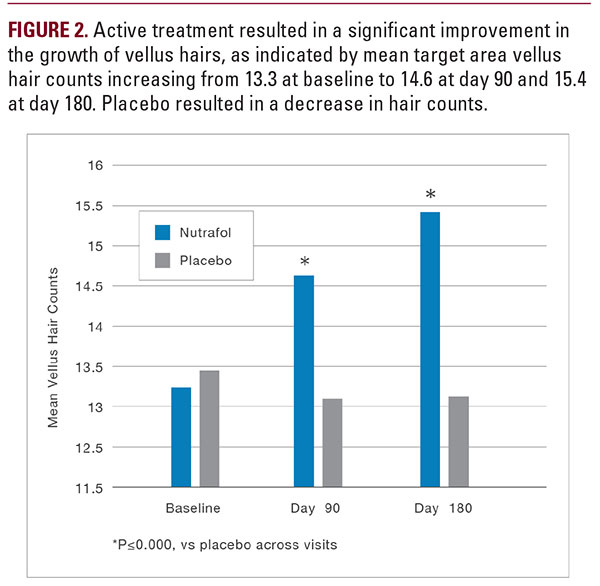
The primary endpoints were increase in terminal, vellus, and total hair counts at 3 and 6 months, as analyzed through phototrichograms. The secondary endpoints were improved grading on the blinded Investigator Global Hair Assessments for hair growth and hair quality, changes in terminal hair diameter and bundle measurements, and responses on the subject SAQ, Ease of Use, and QOL. Safety endpoints were changes in physical exam and queries on potential adverse events (AEs).
Method of Data Analysis
Statistical analysis: Descriptive statistics were obtained for all variables. Tests of normality of continuous measures and homogeneity of variance were performed. Changes from baseline hair growth, hair diameter, hair counts, total score for QOL responses, and SAQ responses were tested using analyses of variance with repeated measurements. Changes in QOL and SAQ items were also tested using Chi-square. All statistical tests were two-tailed. Differences were considered statistically significant at the level of a p value of P less than equal to 0.05.
RESULTS
Demographics and Baseline Characteristics
Subjects in the active treatment group (n=26) and placebo group (n=14) had mean (SD) ages of 48.3 (10.5) years and 53.14 (5.7) years, respectively, which were not significantly different. Two subjects identified themselves as Asian, 32 as Caucasian, and 6 as Hispanic. The 2 groups were not significantly different with respect to race/ethnicity or skin types, and were not different with respect to main study outcomes including the number of terminal hairs in the target area, the number of vellus hairs
Subjects in the active treatment group (n=26) and placebo group (n=14) had mean (SD) ages of 48.3 (10.5) years and 53.14 (5.7) years, respectively, which were not significantly different. Two subjects identified themselves as Asian, 32 as Caucasian, and 6 as Hispanic. The 2 groups were not significantly different with respect to race/ethnicity or skin types, and were not different with respect to main study outcomes including the number of terminal hairs in the target area, the number of vellus hairs






