in the target area, HMI, or mean hair diameter measurements. The 2 groups did not demonstrate any significant differences in lifestyle attributes or baseline mental attitude qualities except for a higher level of stress among subjects in the placebo group (42.3% vs 78.6%; P equals 0.028). All the significant findings reported below were unchanged even after controlling for their initial level of stress using repeated measures (RM)-ANOVA.
Primary Endpoints
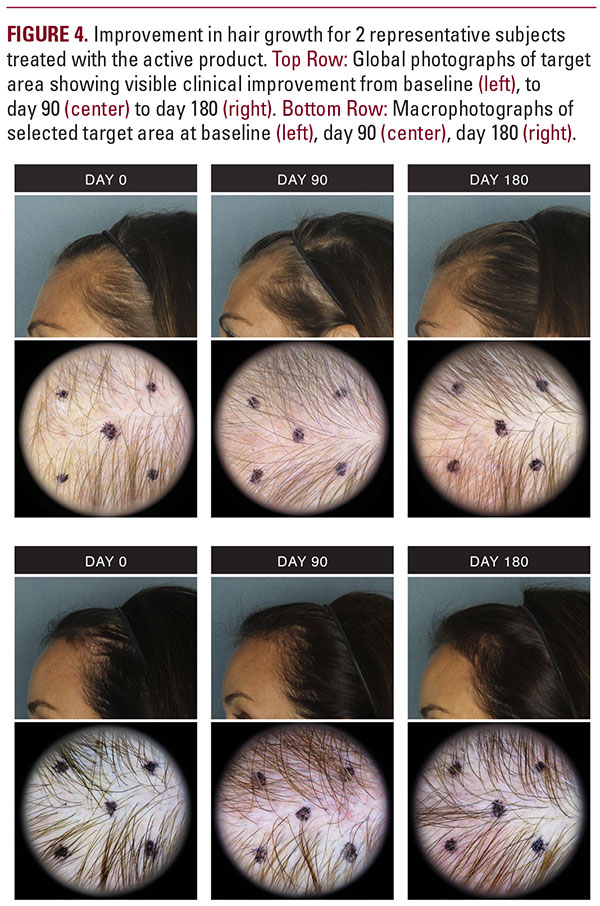
There was a significant difference between groups for the number of terminal (f2,74=6.61, P equals 0.004), vellus (f2,74=12.29, P less than 0.0001), and total hair counts (f2,74=9.11, P less than 0.001) in the target area across all visits. The mean target area hair counts for these endpoints are summarized in Table 3. There were significant improvements at both day 90 and day 180 over baseline for the active group compared with placebo group for these 3 measurements (all P values P less than .009).Figures 1 and 2 demonstrate improvements in terminal and vellus hair growth. The increase in terminal hair counts translated into a significant improvement of 6.8% by day 90 and 10.4% by day 180 from baseline for the active treatment group vs a negligible improvement of 0.07% and 3.5% for the placebo group. For vellus hair counts, the improvement from baseline for the active treatment group was 10.1% by day 90, increasing to 15.7% by day 180. The placebo group resulted in a decrease of vellus hair counts by -2.9% at day 90 and -2.2% at day 180. Similarly, the increase in total hair counts for the active treatment group represented a change of 7.1% at day 90 and 10.8% at day 180 from baseline, while placebo group improvement was negligible at 0.4% and 3% respectively.
Secondary Endpoints
Blinded Investigator Global Hair Assessments
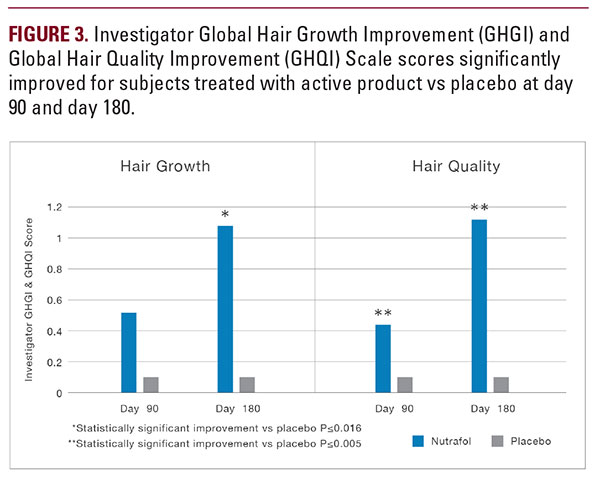
There was a significant and progressive improvement for the active group compared with the placebo group across visits on both






