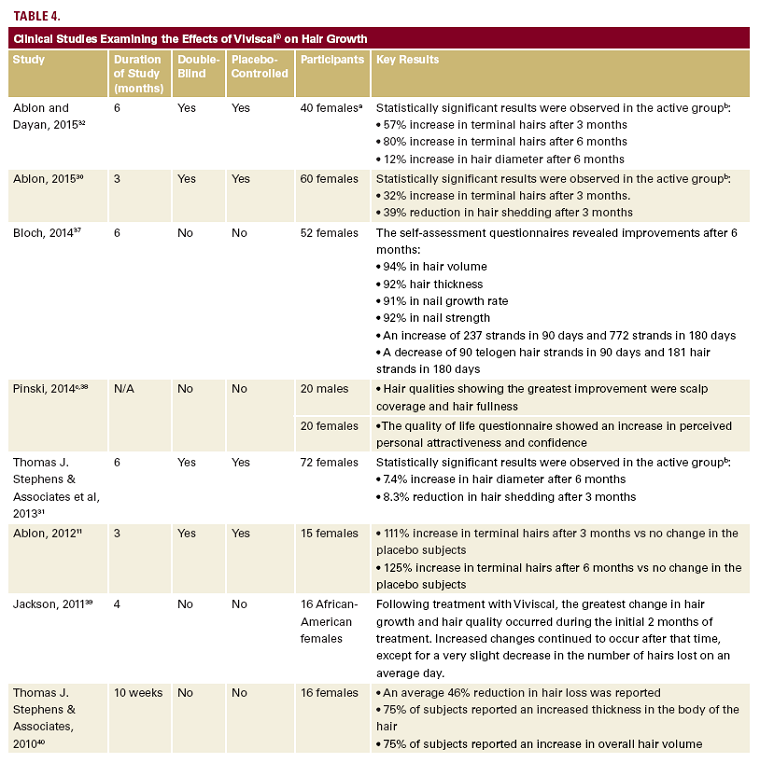
cal. There was no significant change in hair count in the placebo group. Furthermore, the hair diameter also increased by 12% in the treatment group (Table 3). There were also no reported AEs.
Ongoing studies are also seeking to establish the molecular mechanism by which Viviscal promotes hair growth. Results from early in vitro studies have demonstrated that its polysaccharide
complexes have greater bioavailability than similar products (unpublished results). Furthermore, Viviscal has been
shown to enhance the proliferation of dermal papilla (DP) cells, which have been shown to play an important role in orchestrating
the hair growth cycle.33,34 Preliminary studies have illustrated that Viviscal increases the alkaline phosphatase (AP) levels in DP cells (unpublished results). As AP is a key marker of the anagen phase, an increase in its expression suggests an increase in the number of DP cells that are actively growing during the anagen phase.34-36 Thus, in vitro examination of the molecular mechanisms of Viviscal is consistent with the results






