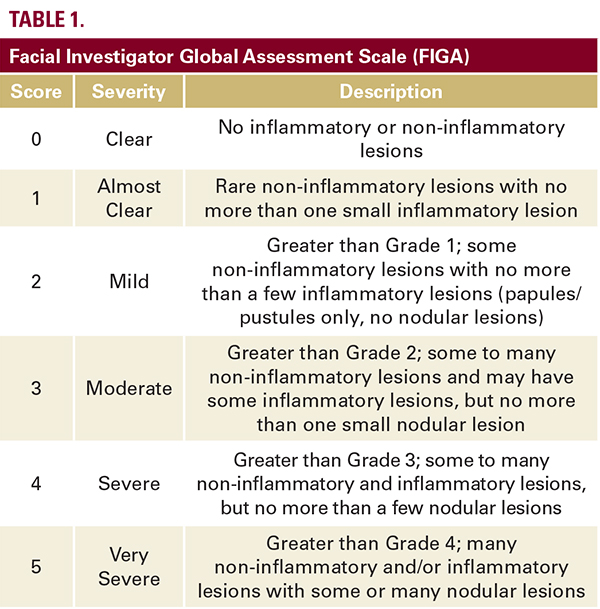
facial acne as defined by a Facial Investigator Global Assessment (FIGA) score of 3 or 4 (Table 1). Subjects were required to be able to understand the requirements of the study and be capable of providing informed consent. Pregnant women, breastfeeding mothers, and females of childbearing potential who were not practicing a reliable method of contraception were excluded from study participation. Females of childbearing potential were required to have a negative urine pregnancy test at baseline and at every study visit, in addition to practicing a reliable method of contraception for the duration of the study. The following exclusionary criteria were also applied: allergy or sensitivity to any component of the test medication, medical conditions that contraindicated participation, skin diseases/disorders that interfered with the diagnosis or evaluation of acne vulgaris, evidence of recent alcohol or drug abuse, history of poor cooperation, non-compliance with medical treatment, or unreliability.Subjects were required to complete the following washout periods: 1 week for over-the-counter acne medications or bleaching agents; 2 weeks for topical therapy with retinoids, antibiotics, benzoyl peroxide, dapsone, bleaching agents, cryotherapy, chemical peels, or microdermabrasion; 4 weeks for oral antibiotics or other investigational drugs; 24 weeks for oral retinoids or laser resurfacing and dermabrasion.The study was performed in accordance with Good Clinical Practices, including guidelines outlined by the International Conference on Harmonisation. Institutional review board approval was obtained from each participating center. Informed consent was obtained from all study participants prior to enrollment.
Treatment
All subjects received azelaic acid 15% foam, a white to off-white hydrophilic emulsion supplied in a 50 g aluminum can pressurized with propellants. Azelaic acid 15% foam contains 15 mg/g of azelaic acid in a vehicle consisting of benzoic acid, cetostearyl alcohol, dimethyl isosorbide, medium-chain triglycerides, methylcellulose, mono- and di-glycerides, polyoxyl 40 stearate, polysorbate 80, propylene glycol, purified water, sodium hydroxide, and xanthan gum.11Subjects were instructed to apply azelaic acid 15% foam sparingly to the entire facial area (cheeks, chin, forehead, and nose) twice daily (morning and evening) and to massage gently into the skin until the foam vanished.
Efficacy Assessments
All subjects were evaluated at baseline before drug administration and at follow-up visits at week 4, week 8, week 12, and week 16. Static assessments of facial acne severity were completed at each visit using the 6-point FIGA, ranging from 0 (clear) to 5 (very severe) (Table 1). The primary efficacy endpoint of this  study was the percent of subjects who achieved Clear or Almost Clear FIGA scores at week 16. Secondary endpoints included the percent reduction of total, inflammatory, and non-inflammatory lesion counts at week 16 as compared to baseline.At the final visit at week 16, subjects were additionally given a “Patient Preference Questionnaire”. This questionnaire asked subjects to rank different treatment vehicles that they had used in the past (gel, lotion, cream, ointment, and spray) from “liked the least” to “liked the best”. Subjects were also asked to compare the study medication against medications that they had used in the past based on ease of use, ability to continue daily activities directly after application, feeling of skin after application, ability to apply to large body surface areas, and absorption. Finally, subjects were asked to rate the study foam on the following qualities: moisturizing, lack of residue, grease, absorption, ease of application, lack of fragrance, spreadability.
study was the percent of subjects who achieved Clear or Almost Clear FIGA scores at week 16. Secondary endpoints included the percent reduction of total, inflammatory, and non-inflammatory lesion counts at week 16 as compared to baseline.At the final visit at week 16, subjects were additionally given a “Patient Preference Questionnaire”. This questionnaire asked subjects to rank different treatment vehicles that they had used in the past (gel, lotion, cream, ointment, and spray) from “liked the least” to “liked the best”. Subjects were also asked to compare the study medication against medications that they had used in the past based on ease of use, ability to continue daily activities directly after application, feeling of skin after application, ability to apply to large body surface areas, and absorption. Finally, subjects were asked to rate the study foam on the following qualities: moisturizing, lack of residue, grease, absorption, ease of application, lack of fragrance, spreadability.
 study was the percent of subjects who achieved Clear or Almost Clear FIGA scores at week 16. Secondary endpoints included the percent reduction of total, inflammatory, and non-inflammatory lesion counts at week 16 as compared to baseline.At the final visit at week 16, subjects were additionally given a “Patient Preference Questionnaire”. This questionnaire asked subjects to rank different treatment vehicles that they had used in the past (gel, lotion, cream, ointment, and spray) from “liked the least” to “liked the best”. Subjects were also asked to compare the study medication against medications that they had used in the past based on ease of use, ability to continue daily activities directly after application, feeling of skin after application, ability to apply to large body surface areas, and absorption. Finally, subjects were asked to rate the study foam on the following qualities: moisturizing, lack of residue, grease, absorption, ease of application, lack of fragrance, spreadability.
study was the percent of subjects who achieved Clear or Almost Clear FIGA scores at week 16. Secondary endpoints included the percent reduction of total, inflammatory, and non-inflammatory lesion counts at week 16 as compared to baseline.At the final visit at week 16, subjects were additionally given a “Patient Preference Questionnaire”. This questionnaire asked subjects to rank different treatment vehicles that they had used in the past (gel, lotion, cream, ointment, and spray) from “liked the least” to “liked the best”. Subjects were also asked to compare the study medication against medications that they had used in the past based on ease of use, ability to continue daily activities directly after application, feeling of skin after application, ability to apply to large body surface areas, and absorption. Finally, subjects were asked to rate the study foam on the following qualities: moisturizing, lack of residue, grease, absorption, ease of application, lack of fragrance, spreadability. Safety and Tolerability
Tolerability was evaluated at baseline before drug administration and at all visits. Investigator assessments of erythema, dryness, peeling, and oiliness severity were rated on a 5-point scale (absent, trace, mild, moderate, severe) (Table 2). Subject assessments of pruritus and burning/stinging severity were rated on a 6-point scale (absent, trace, mild, moderate, marked, severe) (Table 3).Adverse events and concomitant medications or treatments were monitored throughout the study. For each adverse event, including cutaneous and systemic events and any reported subjective skin symptoms, the investigator assessed the duration,






