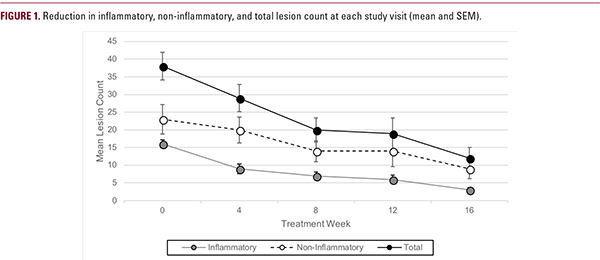
Lesion Counts Significant reductions in mean and median lesion counts occurred within the first 4 weeks of treatment and were sustained or improved throughout the remainder of the study (Figure 1; Table 6). All subjects experienced reductions in inflammatory and total lesion counts by week 16, and 17 of 19 experienced reductions in non-inflammatory lesion counts. Questionnaire When questioned regarding preferences in treatment vehicle, subjects indicated that lotions and creams were generally preferred over gels, ointments, and sprays. Subjects generally preferred the study medication over previously used medications, and most commonly rated its qualities as “good” or “excellent”.
Lesion Counts Significant reductions in mean and median lesion counts occurred within the first 4 weeks of treatment and were sustained or improved throughout the remainder of the study (Figure 1; Table 6). All subjects experienced reductions in inflammatory and total lesion counts by week 16, and 17 of 19 experienced reductions in non-inflammatory lesion counts. Questionnaire When questioned regarding preferences in treatment vehicle, subjects indicated that lotions and creams were generally preferred over gels, ointments, and sprays. Subjects generally preferred the study medication over previously used medications, and most commonly rated its qualities as “good” or “excellent”. Safety and Tolerability
Azelaic acid 15% foam was well tolerated. There were 14 reports of erythema, 3 reports of dryness, 4 reports of peeling, 4 reports of oiliness, 12 reports of pruritus, and 7 reports of burning. There was 1 instance of moderate erythema, 2 instances of moderate pruritus, and 1 instance of moderate burning. All other instances were mild or trace readings, and most instances involved only trace readings that resolved by the end of study. Nine subjects experienced a total of 12 adverse events. Non-serious adverse events that were determined by investigators to be related to the study medication were mild in nature, cutaneous, and occurred at the application site. These included: itching on an eyebrow after drug application (resolved), itching at the application site for 25 minutes (resolved), burning at the application site (unresolved), and a tingling sensation at the application site (resolved). One subject experienced swelling and peeling in the treatment area of moderate severity that was determined to be unlikely to be related to study medication. Study drug application was interrupted for 4 days, and the event resolved with no residual effects. Other non-serious adverse events were mild and determined to be definitely unrelated to study medication included: sunburn, sinus infection, hyperthyroidism, streptococcal pharyngitis, tonsillitis, and