

mg (29.5%-18.6% = 10.9%)12 Since we could not find dose escalation clearing rates for other drugs, this rate also was applied for adalimumab, ixekizumab, and guselkumab dose escalations. Lastly, the rate of complete clearing for switching to another biologic was obtained from previous reported studies.15,16
Cost Considerations
In order to compare the cost per additional patient cleared for the different approaches, we assumed that the cost on the first biologic is their baseline cost and we determined the additional cost.
Adding a Topical Agent
The cost for topical Cal/BD foam (Enstilar, LEO Pharma Inc.), obtained from Medi-Span Price Rx, is $1,050 for 60 g.9 Patients in the study evaluating Cal/BD foam efficacy in psoriasis patients with inadequate response to biologic therapy received Cal/BD foam once daily for 4 weeks.17 The 28% of patients achieved total clearance of plaque psoriasis as early as week 4; we made an assumption that 60 g supply of Cal/BD foam is sufficient to last 4 weeks if applied once daily as reported in the study.18 NNT to effectively clear one additional patient with topical treatment not achieving complete clearance on initial biologic was determined in the same manner as for the dose escalation and switching to another biologic approaches (Figure 1). In order to calculate the cost per additional cleared patient with addition of a topical agent approach, the cost of Cal/BD foam was multiplied by NNT to achieve clearance for one additional patient (Figure 2).
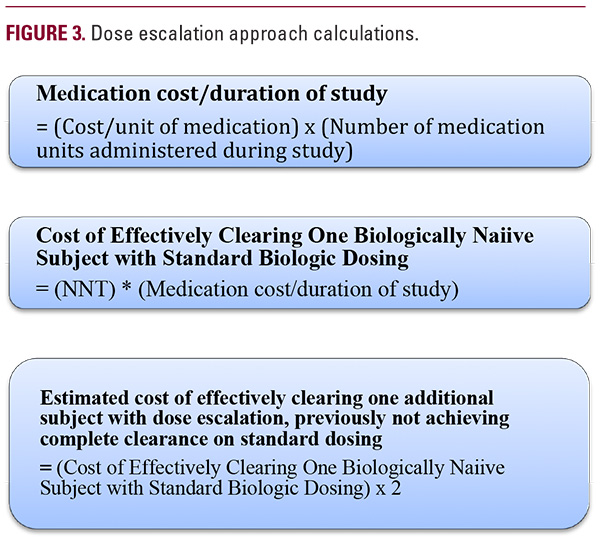
Dose Escalation Approach
Psoriasis clearance data on standard biologic dosing were available for adalimumab, ustekinumab, ixekizumab, and guselkumab.11-14 These studies reported number of subjects and percentage of patients achieving PASI 100 allowing for the calculation of number needed to treat (NNT) to effectively clear one biologically naïve subject with standard biologic dosing (Figure 1). Cost/unit of medication for adalimumab, ustekinumab, ixekizumab, and guselkumab was obtained using available medication wholesale acquisition cost data retrieved from Medi- Span Price Rx.9 Using the description of intervention completed in the studies, we counted the number of medication units administered over the duration of the study. Then, using the cost/ unit of medication and total number of medication units administered during the study, medication cost/duration of study was determined (Figure 3). The next step was to calculate the cost of effectively clearing one biologically naive subject with standard biologic dosing. In order to do this, medication cost/duration of study and previously determined NNT were multiplied (Figure 3). Lastly, to estimate the cost of effectively clearing one additional subject with dose escalation, previously not achieving clearance on standard dosing, we made an assumption that it






