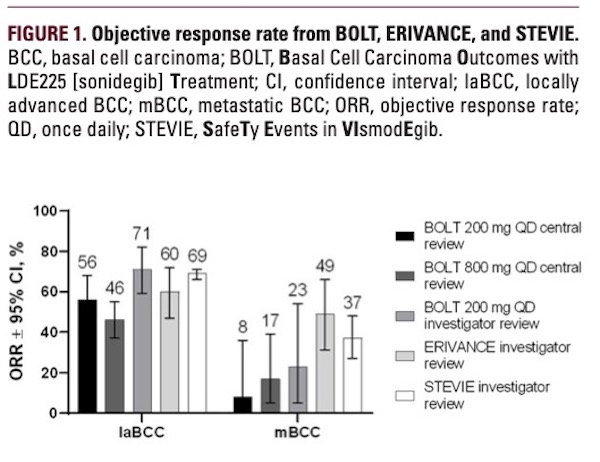
by central review of 56% in laBCC and 8% in mBCC for the 200 mg group, and 46% in laBCC and 17% in mBCC for the 800 mg group (Table 2 and Figure 1). CR by central review reached 5% and 2% for patients with laBCC in the 200 mg and 800 mg groups, respectively, while CR for mBCC was 0% in both dose groups. Investigator-assessed ORR was 71% in laBCC and 23% in mBCC for the 200 mg group, with CR of 9% in laBCC and 0% in mBCC.11 Using RECIST criteria, ORR by central review for patients with laBCC was 59.5% and 55.9% for the 200 and 800 mg groups, respectively, and CR was achieved by 19.0% and 33.3% of patients receiving 200 and 800 mg, respectively.
A total of 96 patients were included in the ERIVANCE efficacy analysis (63 with laBCC and 33 with mBCC), who were 61% male with median age of 62 years.14 Eight patients with laBCC were excluded from the efficacy analysis because the diagnosis was not confirmed by an independent pathologist; these patients were included in the overall population. At 39 months, 8 (8%) patients remained on study treatment; the most common reason for discontinuation was disease progression, seen in 28 (28%) patients.12 The ERIVANCE final analysis reported investigator-assessed ORR of 60% and 49%, including CR of 32% and 0%, for patients with laBCC and mBCC, respectively (Table 2 and Figure 1).12
A secondary analysis of ERIVANCE examined baseline disease severity and clinical benefit of vismodegib treatment in 61
A total of 96 patients were included in the ERIVANCE efficacy analysis (63 with laBCC and 33 with mBCC), who were 61% male with median age of 62 years.14 Eight patients with laBCC were excluded from the efficacy analysis because the diagnosis was not confirmed by an independent pathologist; these patients were included in the overall population. At 39 months, 8 (8%) patients remained on study treatment; the most common reason for discontinuation was disease progression, seen in 28 (28%) patients.12 The ERIVANCE final analysis reported investigator-assessed ORR of 60% and 49%, including CR of 32% and 0%, for patients with laBCC and mBCC, respectively (Table 2 and Figure 1).12
A secondary analysis of ERIVANCE examined baseline disease severity and clinical benefit of vismodegib treatment in 61

patients with laBCC.18 An independent review committee scored lesion photographs on a 5-point scale from 1 (no scarring and no functional impairment at baseline; significant worsening after treatment) to 5 (considerable deformity and functional impairment at baseline; significant clinical benefit after treatment). The majority of patients exhibited severe or moderate disease at baseline (59% scored 5, 13% scored 4), and significant or some clinical benefit after treatment (65% scored 5, 11% scored 4).18






