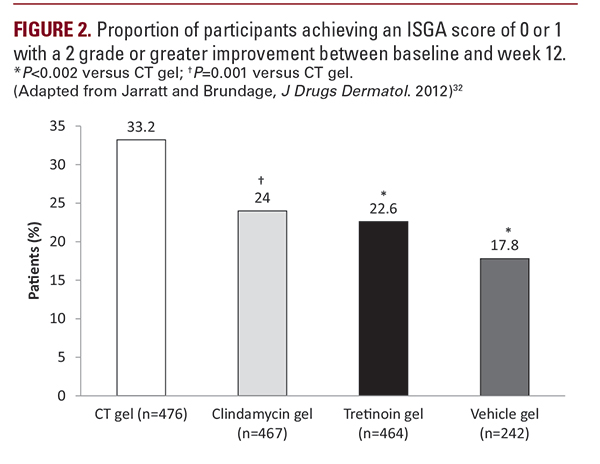
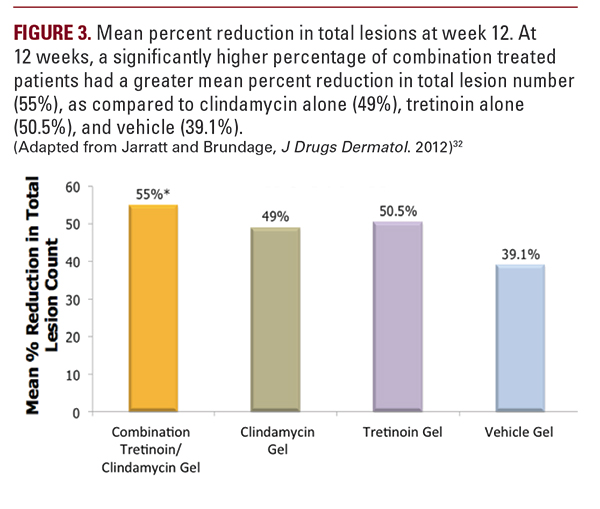
microcomedone formation and extrudes comedones and the topical clindamycin has antibacterial properties and reduces inflammatory lesions. It is also conveniently dosed as a once a day topical application. Combination tretinoin 0.025% and clindamycin phosphate 1.2% has been found to be effective in mild-to-moderate acne vulgaris in 3 pivotal phase 3 studies.29-31 An alcohol-free, aqueous gel formulation of tretinoin 0.025% and clindamycin phosphate 1.2% has since been unveiled.29While clindamycin and tretinoin provide complementary methods of action, they are not readily combined into a single formulation. A combination acne treatment was developed to stabilize and solubilize tretinoin 0.025% and clindamycin phosphate 1.2% in an aqueous-based gel for once-daily treatment of acne vulgaris (Figure 1). The solubilized tretinoin is believed to have a faster rate of cutaneous delivery which may account for the favorable tolerability and low irritation potential.29 One of the essential components of the vehicle is Laureth 4 (polyoxyether of lauryl alcohol). Laureth 4 is a clear, colorless liquid used in the manufacture of cosmetics and personal care products. It functions as both a surfactantand an emulsifier to help solubilize and disperse otherwise non-mixable substances, such as tretinoin and clindamycin.33A multicenter, randomized, double-blind active drug- and vehicle-controlled study, evaluated solubilized tretinoin 0.025%/clindamycin phosphate 1.2% gel compared to its components alone and vehicle alone.32 Those eligible for the study were patients greater than 12 years with active acne vulgaris on the face and an Investigator Global Assessment (IGA) score greater or equal to 2. Exclusion criteria included the following:nodulocystic acne at baseline, pregnant or lactating females, and patients with a history or presence of regional enteritis, inflammatory bowel disease, or history of antibiotic associated colitis.Combined tretinoin 0.025%/clindamycin phosphate 1.2% gel provided a statistically significant improvement in acne as compared to its components alone or the vehicle. At 12 weeks, a higher percentage of combination treated patients achieved anISGA score of 0 or 1 with a 2-grade improvement in IGA (33.2%), compared to clindamycin alone (24%), tretinoin alone (22.6%), and vehicle (17.8%) (Figure 2). Combination treated patients also had a greater mean percent reduction in total lesion number (55%), as compared to clindamycin alone (49%), tretinoin alone (50.5%), and vehicle (39.1%) (Figure 3). The reduction in inflammatory lesions was statistically significant for combination gel (60.4%) versus tretinoin gel (54.5%) and vehicle gel (43.3%), but not when compared to clindamycin gel (56.5%). Reduction in non-inflammatory lesions was statistically significant for combination gel (51%) versus clindamycin gel (42.9%) and vehicle gel (36%), but not compared to tretinoin gel (47.3%).32 In this trial, the combination formulation was found to have excellent tolerability. Observed local treatment-related adverse reactions (>1%) were application site reactions, including dryness, irritation, exfoliation, erythema, pruritus, and dermatitis.Sunburn was also reported. The incidence of skin reactions peaked at 2 weeks and gradually decreased throughout the trial period.32 These findings were consistent with previous reports of minimal adverse events and excellent tolerability of tretinoin gel/clindamycin phosphate formulation in other large, controlled clinical trials, including a 52-week trial.30,31,34
CONCLUSION
Designing vehicle formulations for the topical treatment of acne vulgaris is complex and multifaceted. The optimal formulationmust not only target the multifactorial etiology of acne, but also needs to be effective and well tolerated. This ultimatelytranslates to more rapid clearance of disease and increased