Routine quality control (QC) testing (ie, nitriloacetic acid [NTA], next-generation sequencing [NGS], and multidimensional identification technology [MudPIT]) was performed to determine the quantity, size, miRNA contents, and purity of the exosomes.
Intervention/Preparation of Intervention
Exosomes (Exovex, Exocelbio, Doylestown, PA) are a cell-free preparation in pre-diluted vials of serum at 1 of 4 ready-to-use concentrations: 5 x 109 exosomes in 2 mL of serum, 12 x 109 exosomes in 2.5 mL of serum, 25 x 109 exosomes in 5 mL of serum, and 100 x 109 exosomes in 5 mL of serum. Vials are stored at -20 degrees C until use and must be thawed without shaking before application. In each of the cases detailed below, exosome serum was applied topically. All patients presented here were treated in accordance with the principles outlined in the Declaration of Helsinki, and each patient consented to treatment and photography.
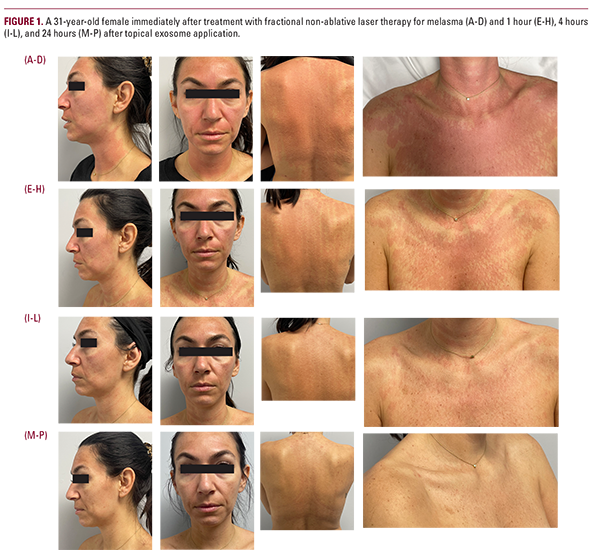
RESULTS
Case Study Patient 1: A 31-year-old woman with acne, mild acne scarring, and melasma who received fractional non-ablative laser treatment (Novel 1,927 nm Fractional Thulium Laser, LaseMD Ultra by Lutronic). The patient received