Literature Searches
Before the expert panel meeting, a systematic literature review was conducted, selecting present clinical guidelines, algorithms, and evidence-based recommendations describing the current best practice in pre-/post-procedure measures for facial laser and energy devices treatment. Additionally, review articles, clinical trials, and other studies were selected that were clinically relevant to the algorithm addressing pre-/ post-procedure measures for facial laser and energy devices treatment. Publications were in the English language dating from 2012 to January 2020. For the literature search, we used the following terms:
Pre-/post-procedure measures for facial laser and energy devices treatment; Guidelines; Algorithm; Adverse events; Complications; Prevention; Pain; Bruising; Swelling; Discoloration; Infection; Reactivation of herpes simplex virus; Antiviral medication; Scarring; Comfort; Sun exposure; Skincare; Wound healing regimen.
Exclusion criteria were lack of original data, information not specific to pre-/post-procedure measures for facial laser and energy devices treatment, and publication in a language other than English. A dermatologist and a physician/scientist conducted the literature searches. Two reviewers independently evaluated the results of the searches. The American Academy of Dermatology grading system was used for categorizing the selected publications.10
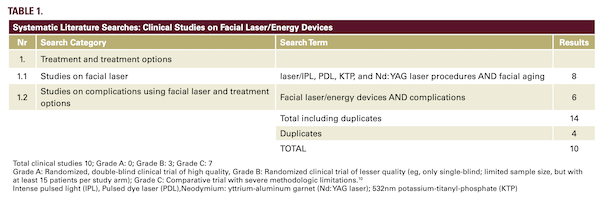
The searches yielded seventy-six articles. After removal of duplicates and those that did not meet the inclusion criteria, twenty-seven articles were included (Figure 1). Of the ten selected clinical studies, three were graded B and seven were graded C (Table 1).10 An additional four papers were selected describing the use of antimicrobial measures.
Before the expert panel meeting, a systematic literature review was conducted, selecting present clinical guidelines, algorithms, and evidence-based recommendations describing the current best practice in pre-/post-procedure measures for facial laser and energy devices treatment. Additionally, review articles, clinical trials, and other studies were selected that were clinically relevant to the algorithm addressing pre-/ post-procedure measures for facial laser and energy devices treatment. Publications were in the English language dating from 2012 to January 2020. For the literature search, we used the following terms:
Pre-/post-procedure measures for facial laser and energy devices treatment; Guidelines; Algorithm; Adverse events; Complications; Prevention; Pain; Bruising; Swelling; Discoloration; Infection; Reactivation of herpes simplex virus; Antiviral medication; Scarring; Comfort; Sun exposure; Skincare; Wound healing regimen.
Exclusion criteria were lack of original data, information not specific to pre-/post-procedure measures for facial laser and energy devices treatment, and publication in a language other than English. A dermatologist and a physician/scientist conducted the literature searches. Two reviewers independently evaluated the results of the searches. The American Academy of Dermatology grading system was used for categorizing the selected publications.10
The searches yielded seventy-six articles. After removal of duplicates and those that did not meet the inclusion criteria, twenty-seven articles were included (Figure 1). Of the ten selected clinical studies, three were graded B and seven were graded C (Table 1).10 An additional four papers were selected describing the use of antimicrobial measures.
RESULTS
The Algorithm
An algorithm is a precise, unambiguous, logical step-by-step method used to solve a problem.9 A clinical algorithm aims to support medical decision making, such as standardizing the selection and use of treatment regimens, thereby improving adherence to evidence-based recommendations. A well-designed algorithm has inputs and outputs, has uniquely defined steps and stops after a finite number of instructions.9
The algorithm for pre-/post-procedure measures for facial laser and energy devices treatment has four sections: prevention, pre-procedure, intra-procedure, and post-procedure.
Section 1: Prevention
The patient should avoid excessive sun exposure before, during, and after treatments as sun exposure can contribute to post-inflammatory changes, or limit the effectiveness of the procedure.5,6,11,12 To protect the treated skin from sun exposure a broad-spectrum sunscreen with an SPF 30, or higher, is to be started at least four weeks prior to the first laser or another energy device treatment.5,6,11,12
The literature supports universal oral antiviral prophylaxis, though, in practice, many clinicians only find this necessary/helpful in patients undergoing ablative procedures.6 It is unclear from the literature what dose to give and when to start prophylactic antiviral treatment.5,6,13-16 Some authors recommend acyclovir (400 mg orally three times daily) or valacyclovir (500 mg orally two times daily), starting one day before the procedure and continuing for 6–10 days post-procedure.5,6,13-16
A survey completed by fifty-six dermatologists and surgeons showed that oral prophylactic antivirals were used before treatment by 96% of the respondents.6 Of the fifty-five
An algorithm is a precise, unambiguous, logical step-by-step method used to solve a problem.9 A clinical algorithm aims to support medical decision making, such as standardizing the selection and use of treatment regimens, thereby improving adherence to evidence-based recommendations. A well-designed algorithm has inputs and outputs, has uniquely defined steps and stops after a finite number of instructions.9
The algorithm for pre-/post-procedure measures for facial laser and energy devices treatment has four sections: prevention, pre-procedure, intra-procedure, and post-procedure.
Section 1: Prevention
The patient should avoid excessive sun exposure before, during, and after treatments as sun exposure can contribute to post-inflammatory changes, or limit the effectiveness of the procedure.5,6,11,12 To protect the treated skin from sun exposure a broad-spectrum sunscreen with an SPF 30, or higher, is to be started at least four weeks prior to the first laser or another energy device treatment.5,6,11,12
The literature supports universal oral antiviral prophylaxis, though, in practice, many clinicians only find this necessary/helpful in patients undergoing ablative procedures.6 It is unclear from the literature what dose to give and when to start prophylactic antiviral treatment.5,6,13-16 Some authors recommend acyclovir (400 mg orally three times daily) or valacyclovir (500 mg orally two times daily), starting one day before the procedure and continuing for 6–10 days post-procedure.5,6,13-16
A survey completed by fifty-six dermatologists and surgeons showed that oral prophylactic antivirals were used before treatment by 96% of the respondents.6 Of the fifty-five






