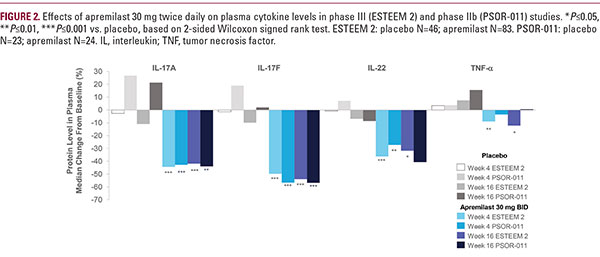
from a subset of patients from the Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis (ESTEEM 2), a phase III study in North America and Europe, as well as from patients in a separate phase IIb study conducted in Japan (PSOR-011).24 In ESTEEM 2, treatment with apremilast 30 mg twice daily was associated with significant reductions inplasma levels of IL-17F, IL-17A, IL-22, and TNF-α compared with placebo as early as week 4 and decreases in cytokine levels were sustained through week 44 (Figure 2).24,25 Similar reductions emerged in patients who were initially randomized to receive placebo and were switched to apremilast treatment at week 16. Findings were generally similar in PSOR-011 (Figure 2).24,25 The rapid, substantial reduction in systemic IL-17 protein levels should be highlighted: In these two studies, the median reduction was approximately −43% to −44% for IL-17A and −50% to −57% for IL-17F at week 4. The median reductions in systemic IL-22 and TNF-α protein levels were significant, although more modest (−27% to −36% for IL-22 and −3% to −9% for TNF-α at week 4).24,25 Further analysis of the ESTEEM subset of patients showed positive correlations between changes from baseline in IL-17A, IL-17F, and IL-22 levels at week 4 and PASI improvement at week 16; only weak correlations were observed between the change in TNF-α and PASI at week 16.24,26 It should be noted that the decrease in IL-17A plasma protein levels in phase IIb and phase III (median −42 to −44% at week 1624,25) is very similar to the observed decrease in IL-17A gene expression in the lesional skin (median −46.7% at week 12) observed in a separate phase II study of apremilast in patients with recalcitrant plaque psoriasis in the United States.23 Therefore, these findings of IL-17 inhibition are consistent and reproducible across multiple studies, are in line with those of previous in vitro investigations, and demonstrate that inhibition of IL-17 is an important mechanism through which apremilast exerts its anti-inflammatory effects in patients with psoriasis. Because apremilast modulates the expression of a numberof cytokines involved in the pathogenesis of psoriasis (ie, IL-17A/F, IL-22, and TNF-α), nonlinear, multivariate algorithms were used in ESTEEM 2 to examine potential interactions and synergies between cytokine levels at week 4 and PASI improvement at week 16. The inclusion of nonlinearities, interactions, and synergies resulted in a greater ability to predict week 16 PASI improvement than was found with linear univariate models. These analyses revealed that IL-17F is the most important predictor of PASI improvement but that synergies between IL-17A/F, IL-22, and TNF- α clearly exist.24 Therefore, the well-characterized pleiotropic effects of apremilast appear to create a unique therapeutic milieu, in which decreases in multiple cytokines are not simply additive, but feed into one another, resulting in observed clinical improvements (Figure 3A and 3B).
Clinical Profile of Apremilast
The efficacy and safety of apremilast in the treatment of moderate to severe psoriasis have been demonstrated in the comprehensive global ESTEEM phase III trial program and the LIBERATE phase IIIb trial.27-29 In the ESTEEM and LIBERATEtrials, adult patients with moderate to severe psoriasis receiving apremilast demonstrated statistically significant and clinically meaningful improvement, as measured by PASI-75 response at week 16, the primary end point.27-29 Other analyses from the ESTEEM trials showed that apremilast treatment significantly reduces pruritus,30 a symptom reported as highly bothersome.4 QOL also significantly improved in the ESTEEM trials, with significant improvements from baseline at week 16 in mean Dermatology Life Quality Index scores in patients treated with apremilast versus placebo.27,28 At week 16, the most commonly occurring adverse events in ESTEEM patients taking apremilast were diarrhea, nausea, upper respiratory tract infection, and headache.27,28 Apremilast demonstrated