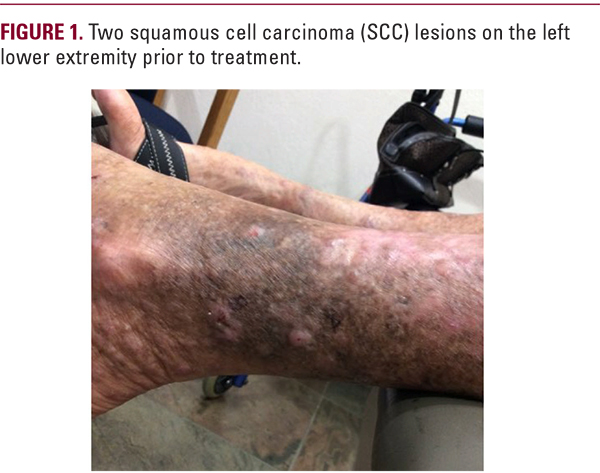
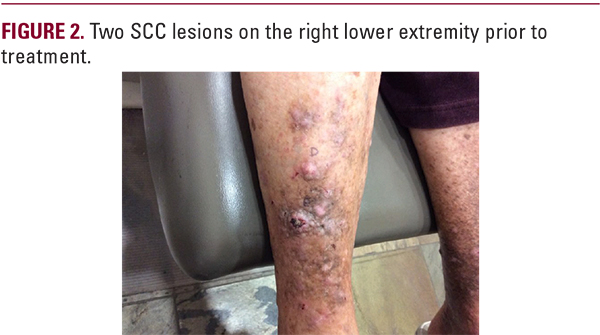

DISCUSSION
First-line treatment for NMSC remains surgical intervention, however in situations in which patients are poor surgical candidates, other modalities must be employed. Factors implicated in unfavorable surgical outcomes which lead to prolonged wound healing include a history of hip or knee replacement surgery, an ankle brachial index of less than 0.80, female gender, elderly age, and increased initial lesion size.4 Further exploration of non-invasive treatment modalities must be explored for this patient population as to not lead to chronic ulcerations. Intralesional therapy with 5-Fluorouracil is an inexpensive, efficacious, and non-invasive method with which to approach these lesions.3 The cost of a 50-mL vial of 5-Fluorouracil is cited between $19.50-$26.00, with the treatment regimen employed in this case report, up to thirteen lesions could be treated per vial.5-Fluorouracil’s inhibition of thymidylate synthetase and subsequent inhibition of DNA synthesis has led it to become widely utilized in treatment of various malignancies. However, intralesional 5-fluorouracil for NMSC remains a relatively uninvestigated area of non-invasive treatment. This has led to a reduced usage of intralesional therapy, potentially due to the absence of therapeutic guidelines and the existence of few well-designed clinical trials.5 While the few clinical trials performed have revealed promising results, more investigation is warranted.A clinical trial performed in 1997 by Miller et al6 revealed a 91% biopsy-proven cure rate of basal cell carcinoma in patients treated with 0.5 mL of a gel consisting of 5-fluorouracil (30 mg/ mL), epinephrine (0.1 mg/mL), and bovine collagen. Treatments regimen consisted of injections of the gel for three times a week for two successive weeks. 5-Fluorouracil has also been implicated in the treatment of squamous cell carcinoma with initial reports of this treatment modality in 1962.7 A clinical trial consisting of 25 patients reported a 96% cure rate of well-differentiated squamous cell carcinomas. The treatment regimen in this study consisted of 4-6 injections of 1 mL of 5-fluorouracil (30mg/mL). Patients in this study reported good overall cosmesis and no significant side effects.6 While most data sets reporting on 5-fluorouracil’s intralesional efficacy in NMSC are relatively small they generally show superiority in comparison to the other intralesional agents.7 While not directly studied head to head, multiple studies have reported superior outcomes of 5-fluorouracil in comparison to methotrexate in the treatment of keratoacanthomas.3,8 Meta-analysis of other intralesional therapies has shown 5-fluorouracil to have superior cure rates for NMSC in comparison to interferon and interleukin-2.7 In conclusion, intralesional use of 5-fluorouracil in NMSC is an inexpensive, efficacious, and non-invasive method of treatment for non-surgical candidates. With the advent of further additional clinical trials and large-scale studies a more pin-point set of clinical guidelines may be created. With greater data sets the ability to determine the optimal dosage and interval of treatment will be elucidated and allow for better future treatment with this modality.
DISCLOSURE
The authors have no conflicts.






