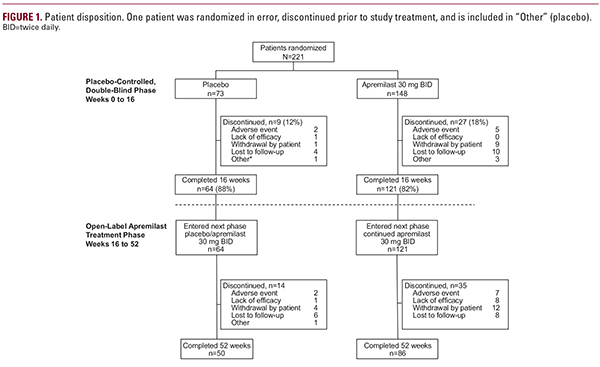
 groups, respectively. Also at baseline, mean psoriasis-involved BSA was 7.2%, mean PASI score was 8.1, mean PGAxBSA score was 21.8, and mean DLQI score was 11.0. Most patients (82%) reported prior treatment with topical therapy. In addition, 167 (76%) patients had scalp disease and 83 (38%) patients had nail disease at baseline.10Efficacy AssessmentsAt week 16, mean percentage change from baseline in PGAxBSA score (primary end point) was significantly greater with apremilast vs. placebo (−48.1% vs. −10.2%; P less than 0.0001).10 At week 52, improvements in all efficacy end points were maintained among patients in the apremilast/apremilast group and emerged in patients in the placebo/apremilast group after switching to apremilast (Table 1). Mean percentage improvement in PGAxBSA score was sustained among patients who continued receiving apremilast through week 52 (Figure 2). In the apremilast/apremilast group, mean percentage change from baseline in PGAxBSA score at week 52 was −55.5% (Table 1); patients in the placebo/apremilast group demonstrated improvements at week 52 (−42.2%). Achievement of PGAxBSA-75 was maintained or improved during the open-label treatment phase (Figure 3). Specifically, 42.1% of patients initially randomized to apremilast and 45.3% initially randomized to placebo achieved PGAxBSA-75 at week 52 (Table 1). Similarly, proportions of patients with sPGA score of 0 (clear) or 1 (almost clear) were maintained or improved in the apremilast/apremilast and placebo/apremilast groups, respectively, during open-label apremilast treatment (Table 1).At week 52, 37.5% of patients in the placebo/apremilast group and 26.4% in the apremilast/apremilast group achieved PASI-75 (Table 1). Improvements in pruritus VAS score and scalp and nail disease (ie, ScPGA and NAPSI scores) were maintained in the apremilast/apremilast group and emerged in the placebo/apremilast group during open-label treatment with apremilast (Figure 4; Table 1).QOL AssessmentsDLQI score improvements observed during the placebo-controlled period10 were sustained over 52 weeks among patients in the apremilast/apremilast group (−4.4), and improvements emerged among patients in the placebo/apremilast group (−5.1; Table 1). Among patients with baseline DLQI score >5, the proportion of patients who achieved DLQI MCID was maintained in patients continuing apremilast for up to 52 weeks, and increased after patients were switched to apremilast (Figure 5).Satisfaction scores at week 52 for apremilast based on the TSQM, version II, indicated high levels of satisfaction based on domain scores for effectiveness, convenience, side effects, and global satisfaction. At week 52, patients reported high satisfaction on the
groups, respectively. Also at baseline, mean psoriasis-involved BSA was 7.2%, mean PASI score was 8.1, mean PGAxBSA score was 21.8, and mean DLQI score was 11.0. Most patients (82%) reported prior treatment with topical therapy. In addition, 167 (76%) patients had scalp disease and 83 (38%) patients had nail disease at baseline.10Efficacy AssessmentsAt week 16, mean percentage change from baseline in PGAxBSA score (primary end point) was significantly greater with apremilast vs. placebo (−48.1% vs. −10.2%; P less than 0.0001).10 At week 52, improvements in all efficacy end points were maintained among patients in the apremilast/apremilast group and emerged in patients in the placebo/apremilast group after switching to apremilast (Table 1). Mean percentage improvement in PGAxBSA score was sustained among patients who continued receiving apremilast through week 52 (Figure 2). In the apremilast/apremilast group, mean percentage change from baseline in PGAxBSA score at week 52 was −55.5% (Table 1); patients in the placebo/apremilast group demonstrated improvements at week 52 (−42.2%). Achievement of PGAxBSA-75 was maintained or improved during the open-label treatment phase (Figure 3). Specifically, 42.1% of patients initially randomized to apremilast and 45.3% initially randomized to placebo achieved PGAxBSA-75 at week 52 (Table 1). Similarly, proportions of patients with sPGA score of 0 (clear) or 1 (almost clear) were maintained or improved in the apremilast/apremilast and placebo/apremilast groups, respectively, during open-label apremilast treatment (Table 1).At week 52, 37.5% of patients in the placebo/apremilast group and 26.4% in the apremilast/apremilast group achieved PASI-75 (Table 1). Improvements in pruritus VAS score and scalp and nail disease (ie, ScPGA and NAPSI scores) were maintained in the apremilast/apremilast group and emerged in the placebo/apremilast group during open-label treatment with apremilast (Figure 4; Table 1).QOL AssessmentsDLQI score improvements observed during the placebo-controlled period10 were sustained over 52 weeks among patients in the apremilast/apremilast group (−4.4), and improvements emerged among patients in the placebo/apremilast group (−5.1; Table 1). Among patients with baseline DLQI score >5, the proportion of patients who achieved DLQI MCID was maintained in patients continuing apremilast for up to 52 weeks, and increased after patients were switched to apremilast (Figure 5).Satisfaction scores at week 52 for apremilast based on the TSQM, version II, indicated high levels of satisfaction based on domain scores for effectiveness, convenience, side effects, and global satisfaction. At week 52, patients reported high satisfaction on theEfficacy and Safety of Apremilast in Systemic- and Biologic-Naive Patients With Moderate Plaque Psoriasis: 52-Week Results of UNVEIL
February 2018 | Volume 17 | Issue 2 | Original Article | 221 | Copyright © February 2018
Linda Stein Gold MD,a Jerry Bagel MD,b Mark Lebwohl MD,c J. Mark Jackson MD,d Rongdean Chen PhD,e Joana Goncalves MD,e Eugenia Levi PharmD,e Kristina Callis Duffin MD MSf
aHenry Ford Health System, West Bloomfield, MI bPsoriasis Treatment Center of Central New Jersey, East Windsor, NJ cIcahn School of Medicine at Mount Sinai, New York, NY dUniversity of Louisville, Forefront Dermatology, Louisville, KY eCelgene Corporation, Summit, NJ fUniversity of Utah, Salt Lake City, UT
 groups, respectively. Also at baseline, mean psoriasis-involved BSA was 7.2%, mean PASI score was 8.1, mean PGAxBSA score was 21.8, and mean DLQI score was 11.0. Most patients (82%) reported prior treatment with topical therapy. In addition, 167 (76%) patients had scalp disease and 83 (38%) patients had nail disease at baseline.10Efficacy AssessmentsAt week 16, mean percentage change from baseline in PGAxBSA score (primary end point) was significantly greater with apremilast vs. placebo (−48.1% vs. −10.2%; P less than 0.0001).10 At week 52, improvements in all efficacy end points were maintained among patients in the apremilast/apremilast group and emerged in patients in the placebo/apremilast group after switching to apremilast (Table 1). Mean percentage improvement in PGAxBSA score was sustained among patients who continued receiving apremilast through week 52 (Figure 2). In the apremilast/apremilast group, mean percentage change from baseline in PGAxBSA score at week 52 was −55.5% (Table 1); patients in the placebo/apremilast group demonstrated improvements at week 52 (−42.2%). Achievement of PGAxBSA-75 was maintained or improved during the open-label treatment phase (Figure 3). Specifically, 42.1% of patients initially randomized to apremilast and 45.3% initially randomized to placebo achieved PGAxBSA-75 at week 52 (Table 1). Similarly, proportions of patients with sPGA score of 0 (clear) or 1 (almost clear) were maintained or improved in the apremilast/apremilast and placebo/apremilast groups, respectively, during open-label apremilast treatment (Table 1).At week 52, 37.5% of patients in the placebo/apremilast group and 26.4% in the apremilast/apremilast group achieved PASI-75 (Table 1). Improvements in pruritus VAS score and scalp and nail disease (ie, ScPGA and NAPSI scores) were maintained in the apremilast/apremilast group and emerged in the placebo/apremilast group during open-label treatment with apremilast (Figure 4; Table 1).QOL AssessmentsDLQI score improvements observed during the placebo-controlled period10 were sustained over 52 weeks among patients in the apremilast/apremilast group (−4.4), and improvements emerged among patients in the placebo/apremilast group (−5.1; Table 1). Among patients with baseline DLQI score >5, the proportion of patients who achieved DLQI MCID was maintained in patients continuing apremilast for up to 52 weeks, and increased after patients were switched to apremilast (Figure 5).Satisfaction scores at week 52 for apremilast based on the TSQM, version II, indicated high levels of satisfaction based on domain scores for effectiveness, convenience, side effects, and global satisfaction. At week 52, patients reported high satisfaction on the
groups, respectively. Also at baseline, mean psoriasis-involved BSA was 7.2%, mean PASI score was 8.1, mean PGAxBSA score was 21.8, and mean DLQI score was 11.0. Most patients (82%) reported prior treatment with topical therapy. In addition, 167 (76%) patients had scalp disease and 83 (38%) patients had nail disease at baseline.10Efficacy AssessmentsAt week 16, mean percentage change from baseline in PGAxBSA score (primary end point) was significantly greater with apremilast vs. placebo (−48.1% vs. −10.2%; P less than 0.0001).10 At week 52, improvements in all efficacy end points were maintained among patients in the apremilast/apremilast group and emerged in patients in the placebo/apremilast group after switching to apremilast (Table 1). Mean percentage improvement in PGAxBSA score was sustained among patients who continued receiving apremilast through week 52 (Figure 2). In the apremilast/apremilast group, mean percentage change from baseline in PGAxBSA score at week 52 was −55.5% (Table 1); patients in the placebo/apremilast group demonstrated improvements at week 52 (−42.2%). Achievement of PGAxBSA-75 was maintained or improved during the open-label treatment phase (Figure 3). Specifically, 42.1% of patients initially randomized to apremilast and 45.3% initially randomized to placebo achieved PGAxBSA-75 at week 52 (Table 1). Similarly, proportions of patients with sPGA score of 0 (clear) or 1 (almost clear) were maintained or improved in the apremilast/apremilast and placebo/apremilast groups, respectively, during open-label apremilast treatment (Table 1).At week 52, 37.5% of patients in the placebo/apremilast group and 26.4% in the apremilast/apremilast group achieved PASI-75 (Table 1). Improvements in pruritus VAS score and scalp and nail disease (ie, ScPGA and NAPSI scores) were maintained in the apremilast/apremilast group and emerged in the placebo/apremilast group during open-label treatment with apremilast (Figure 4; Table 1).QOL AssessmentsDLQI score improvements observed during the placebo-controlled period10 were sustained over 52 weeks among patients in the apremilast/apremilast group (−4.4), and improvements emerged among patients in the placebo/apremilast group (−5.1; Table 1). Among patients with baseline DLQI score >5, the proportion of patients who achieved DLQI MCID was maintained in patients continuing apremilast for up to 52 weeks, and increased after patients were switched to apremilast (Figure 5).Satisfaction scores at week 52 for apremilast based on the TSQM, version II, indicated high levels of satisfaction based on domain scores for effectiveness, convenience, side effects, and global satisfaction. At week 52, patients reported high satisfaction on the





