 with moderate disease and should be incorporated into future studies in this patient population. The safety pro le of apremilast in the UNVEIL patient population was similar to that observed previously in phase III trials.11,12,17-19 Of note, rates of diarrhea (16.4%), headache (11.0%), and nausea (9.6%) in the placebo group in UNVEIL were higher than those in the placebo groups of the ESTEEM studies (diarrhea, ~6.1%; headache, ~2.6%; nausea, ~6.6%). Rates of diarrhea in UNVEIL were higher with apremilast treatment (~29%) than in other randomized, controlled trials of apremilast (15% to 19%).11,12,17-19 This is likely due to proactive questioning of patients reporting diarrhea in this trial (which was not done in ESTEEM) and the fact that, among physicians, diarrhea is now a widely known AE with apremilast, which may have resulted in hypervigilance in reporting. With follow-up questioning, protocol-defined diarrhea (≥2 loose watery stools in 1 day) was confirmed in 19% of patients receiving apremilast, which is more in line with reported rates.11,12,17-19 Changes in laboratory parameters were transient and not clinically significant, consistent with previous studies,11,12 confirming that apremilast does not require regular blood monitoring.
with moderate disease and should be incorporated into future studies in this patient population. The safety pro le of apremilast in the UNVEIL patient population was similar to that observed previously in phase III trials.11,12,17-19 Of note, rates of diarrhea (16.4%), headache (11.0%), and nausea (9.6%) in the placebo group in UNVEIL were higher than those in the placebo groups of the ESTEEM studies (diarrhea, ~6.1%; headache, ~2.6%; nausea, ~6.6%). Rates of diarrhea in UNVEIL were higher with apremilast treatment (~29%) than in other randomized, controlled trials of apremilast (15% to 19%).11,12,17-19 This is likely due to proactive questioning of patients reporting diarrhea in this trial (which was not done in ESTEEM) and the fact that, among physicians, diarrhea is now a widely known AE with apremilast, which may have resulted in hypervigilance in reporting. With follow-up questioning, protocol-defined diarrhea (≥2 loose watery stools in 1 day) was confirmed in 19% of patients receiving apremilast, which is more in line with reported rates.11,12,17-19 Changes in laboratory parameters were transient and not clinically significant, consistent with previous studies,11,12 confirming that apremilast does not require regular blood monitoring. CONCLUSION
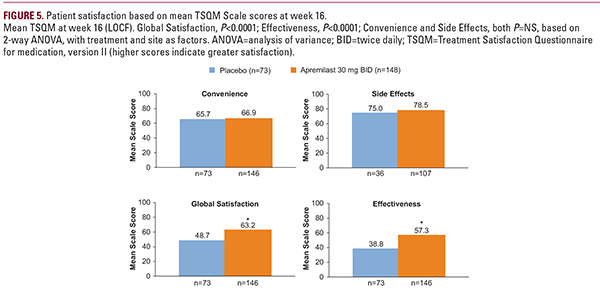
Apremilast was effective in the treatment of systemic-naive, post-topical patients with more moderate plaque psoriasis and was generally well tolerated. The efficacy and safety of apremilast demonstrated in UNVEIL are consistent with those seen in patients with moderate to severe plaque psoriasis in randomized phase III trials.11,12 UNVEIL demonstrated a significantly positive impact of apremilast on QOL and treatment satisfaction in patients with more moderate psoriasis.
DISCLOSURES
Bruce Strober MD PhD has received honoraria for serving as a consultant and advisory board member for AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Celgene Corporation, Dermira, Eli Lilly, Forward Pharma, Janssen, LEO Pharma, Maruho, Medac, Novartis, Pfizer, Stiefel/GlaxoSmithKline, Sun Pharma, and UCB; has received payments (to the University of Connecticut) as an investigator for AbbVie, Amgen, Celgene Corporation, Eli Lilly, Janssen, Merck, Novartis, and Pfizer; has received fees as a scientific director for the CORRONA Psoriasis Registry; and has received grant support (to the University of Connecticut for Fellowship Program) from AbbVie and Janssen. Jerry Bagel MD is a speaker board member, consultant, and/or reports research support from AbbVie, Amgen, Boehringer Ingelheim, Janssen, LEO Pharma, Eli Lilly, Novartis, Pfizer, and Valeant. Mark Lebwohl MD is an employee of Mount Sinai, which receives funds from Boehringer Ingelheim, Celgene Corporation, Eli Lilly, Janssen/Johnson & Johnson, Kadmon, MedImmune/ AstraZeneca, Novartis, Pfizer, and ViDac. Linda Stein Gold MD is investigator and/or consultant for Celgene Corporation, LEO Pharma, Novartis, Pfizer, and Stiefel/GlaxoSmithKline. J. Mark Jackson MD has received research, honoraria, consulting, and/or other support from AbbVie, Amgen, Celgene Corporation, Dermira, Galderma, Genentech, Janssen, Lilly, Medimetriks, Merck, Novartis, Pfizer, Promius, and TopMD.






