have demonstrated skin quality improvements and recipient-reported emotional and functional benefits, including elevated self-esteem and confidence.10-12 Based upon current evidence, expert recommendations support the use of PLLA-SCA for facial rejuvenation, according to the approved indication.13-15
Since 2004, this product has been approved in the US for restoration and/or correction of signs of facial fat loss (lipoatrophy) in people with human immunodeficiency virus, and since 2009 also for correction of shallow to deep nasolabial fold contour deficiencies and other facial wrinkles in immune-competent individuals.5 Recently (2023), the US FDA approved an extension of the indication to include the correction of fine lines and wrinkles in the cheek region for use in immune-competent subjects, based on the study results presented here.
This study evaluated the effectiveness and safety of the PLLA-SCA injectable implant in the correction of cheek wrinkles compared with a no-treatment control, using the preparation and administration protocol published by Palm et al (2021), in which treatment was administered immediately after PLLA-SCA reconstitution in sterile water for injection (SWFI; 8 mL) + 1 mL lidocaine solution (2%), rather than waiting the standard 2 hours before injection.5,16-18 The adapted protocol was intended to support safety and tolerability outcomes with PLLA-SCA, and to aid convenience for physicians.
MATERIALS AND METHODS
Study Design
A randomized, evaluator-blinded, no-treatment controlled study was conducted between November 2019 and August 2021 at 13 sites in the US to assess the effectiveness and safety of PLLA-SCA injections for the correction of cheek wrinkles (NCT04124692). The study was conducted in accordance with the Declaration of Helsinki and the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice as applicable for medical devices. Subjects gave written informed consent and ethical approval was obtained from each relevant institutional review board.
Live study assessments were conducted by blinded evaluators and treating investigators, and subject self-assessment data were reported via questionnaires and subject diaries. During screening and throughout the study, the validated 5-point Galderma Cheek Wrinkles Scale (GCWS; none, mild, moderate, severe, or very severe) was used to grade the severity of wrinkles in repose (GCWS at rest) and when adopting a closed maximum smile (GCWS dynamic).
Study Population
The study included male/female immune-competent adults (aged >21 years) with cheek wrinkles graded as moderate or severe on each side of the face according to GCWS at rest assessments (blinded evaluator and treating investigator). The difference in wrinkle severity was no more than 1-grade between sides. Individuals who had known allergy to injectable PLLA-SCA or lidocaine had undergone previous tissue augmentation, contouring, resurfacing, or similar therapies, or had facial lesions in the treatment area were excluded. Subjects who were pregnant, planning a pregnancy, or breastfeeding were not allowed to enter the study.
Study Treatment
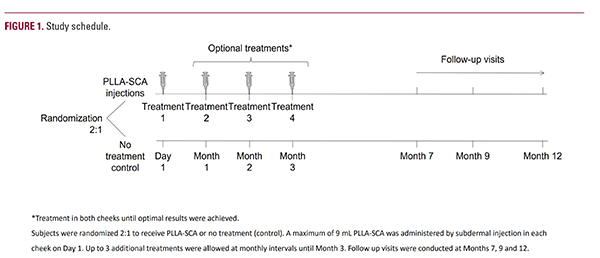
Figure 1 shows the study schedule. Eligible subjects were randomized 2:1 to receive either PLLA-SCA injections (PLLA-SCA group) or no treatment (control group). PLLA-SCA injections were administered on day 1/baseline (Treatment 1). Up to 3 additional