DISCUSSION
The evidence for dupilumab use beyond atopic dermatitis in dermatology is limited, without any current published randomized controlled trials supporting efficacy for additional indications. Retrospective reviews suggest it may be helpful for allergic contact dermatitis and hand dermatitis. Several other reports identify potential uses including alopecia areata and CSU. With small case series and case reports, it is possible that there is positive selection bias for description of successful off-label use of dupilumab in dermatology, and real-world data on off-label dupilumab use overall is lacking.
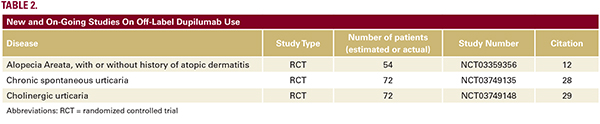
However, this is a promising new drug that may eventually be shown to have efficacy for various conditions in dermatology. Reasonable potential applications include diseases where Th2 responses are important to disease pathophysiology and where an appreciable proportion of patients fail to respond to current therapies, such as chronic urticaria.30 The case series of 6 patients with CSU who failed omalizumab but responded to dupilumab is highly interesting. If dupilumab is shown to be effective for CSU in the RCT currently in progress, many interesting questions follow. For example, CSU patients with low baseline serum total IgE levels tend to poorly respond to omalizumab, but whether response of chronic urticaria to dupilumab varies depending on baseline IgE levels remains to be answered.31
Dupilumab has a relatively safe side effect profile, and the increased rates of conjunctivitis seen with dupilumab use thus far have only been observed in patients with atopic dermatitis.32,33 Its potential for further applications in dermatology in recalcitrant cases is a highly interesting topic, although cost is a significant limiting factor. Ultimately, many further clinical studies, and increased understanding of skin disease pathophysiology will help determine the best possible uses for dupilumab in dermatology.
DISCLOSURES
REFERENCES
- Simpson EL et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335-2348. doi:10.1056/NEJMoa1610020
- Blauvelt A et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287-2303. doi:https://doi. org/10.1016/S0140-6736(17)31191-1
- Bruin-Weller M et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical t. Br J Dermatol. 2017;178(5):1083-1101. doi:10.1111/bjd.16156
- Herz S, Petri M, Sondermann W. New alcohol flushing in a patient with atopic dermatitis under therapy with dupilumab. Dermatol Ther. 2018;0(0):e12762. doi:10.1111/dth.12762
- Flanagan K, Sperling L, Lin J. Drug-induced alopecia after dupilumab therapy. JAAD Case Reports. 2018;5(1):54-56. doi:10.1016/j.jdcr.2018.10.010
- Al Hammadi A, Parmar N V. Erythema, pruritus, and diffuse peeling of skin during dupilumab therapy for atopic dermatitis in three adults. Int J Dermatol. 2019;58(1):e14-e15. doi:10.1111/ijd.14296
- Chipalkatti N et al. A retrospective review of dupilumab for atopic dermatitis patients with allergic contact dermatitis. J Am Acad Dermatol. January 2019. doi:10.1016/j.jaad.2018.12.048
- Machler BC, Sung CT, Darwin E, Jacob SE. Dupilumab use in allergic contact dermatitis. J Am Acad Dermatol. 2019;80(1):280-281.e1. doi:10.1016/j. jaad.2018.07.043
- Lee N et al. A retrospective review of dupilumab for hand dermatitis. Dermatology. 2019:1-2. doi:10.1159/000496481
- Zirwas MJ. Dupilumab for hand eczema. J Am Acad Dermatol. 2018;79(1):167- 169. doi:https://doi.org/10.1016/j.jaad.2018.02.073
- Lee JK, Simpson RS. Dupilumab as a novel therapy for difficult to treat chronic spontaneous urticaria. J Allergy Clin Immunol Pract. 2018. doi:https://doi. org/10.1016/j.jaip.2018.11.018
- ClinicalTrials.gov. Defining reversal of alopecia areata (aa) phenotype with dupilumab in patients with and without associated atopic dermatitis (AD): NCT03359356. Bethesda (MD): National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT03359356. Published 2018. Accessed January 2, 2019.
- Chipalkatti N et al. Dupilumab as a treatment for allergic contact dermatitis. Dermat contact, atopic, Occup drug. 2018;29(6):347-348. doi:10.1097/ DER.0000000000000414
- Goldminz AM, Scheinman PL. A case series of dupilumab-treated allergic contact dermatitis patients. Dermatol Ther. 2018;31(6):e12701. doi:10.1111/ dth.12701
- Joshi SR, Khan DA. Effective use of dupilumab in managing systemic allergic contact dermatitis. Dermat Contact, atopic, Occup drug. 2018;29(5):282- 284. doi:10.1097/DER.0000000000000409
- Oosterhaven JAF, Romeijn GLE, Schuttelaar MLA. Dupilumab treatment of very severe refractory atopic hand eczema. JAMA Dermatol. 2018;154(8):969-970. doi:10.1001/jamadermatol.2018.2027
- Weston GK, Hooper J, Strober BE. Dupilumab in the treatment of dyshidrosis: a report of two cases. J Drugs Dermatol. 2018;17(3):355-356.
- Nanda S, Nagrani N, MacQuhae F, Nichols A. A case of complete resolution of severe plantar dyshidrotic eczema with dupilumab. J Drugs Dermatol. 2019;18(2):211.
- Penzi L et al. Hair regrowth in a patient with long-standing alopecia totalis and atopic dermatitis treated with dupilumab. JAMA Dermatology. 2018;154(11):1358-1360. http://dx.doi.org/10.1001/jamadermatol.2018.2976.
- Alniemi DT, McGevna L. Dupilumab treatment for atopic dermatitis leading to unexpected treatment for alopecia universalis. JAAD Case Reports. 2019;5(2):111-112. doi:10.1016/j.jdcr.2018.11.006
- Smogorzewski J, Sierro T, Compoginis G, Kim G. Remission of alopecia universalis in a patient with atopic dermatitis treated with dupilumab. JAAD Case Reports. 2019;5(2):116-117. doi:10.1016/j.jdcr.2018.11.007
- Darrigade A-S et al. Dual efficacy of dupilumab in a patient with concomitant atopic dermatitis and alopecia areata. Br J Dermatol. 2018;179(2):534-536. doi:10.1111/bjd.16711
- Kaye A et al. Dupilumab for the treatment of recalcitrant bullous pemphigoid. JAMA Dermatol. 2018;154(10):1225-1226. doi:10.1001/jamadermatol.2018.2526
- Gordon SC et al. Eosinophilic annular erythema treated with dupilumab. Pediatr Dermatol. 2018;35(4):e255-e256. doi:10.1111/pde.13533
- Beck KM et al. Dupilumab treatment for generalized prurigo nodularisdupilumab treatment for generalized prurigo nodularis letters. JAMA Dermatol. 2019;155(1):118-120. doi:10.1001/jamadermatol.2018.3912
- Mollanazar NK et al. Reduced itch associated with dupilumab treatment in 4 patients with prurigo nodularis. JAMA Dermatol. November 2018. doi:10.1001/jamadermatol.2018.3906
- Yang EJ, Murase JE. Recalcitrant anal and genital pruritus treated with dupilumab. Int J Women’s Dermatol. 2018;4(4):223-226. doi:10.1016/j. ijwd.2018.08.010
- ClinicalTrials.gov. A multicenter, randomized, double-blind, placebo-controlled, proof-of-concept phase 2, 16-week treatment study with a 16 week follow-up period to assess the efficacy and safety of dupilumab (anti-il4ra) in adult patients with chronic spontaneous urticaria. Bethesda (MD): National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT03749135. Published 2018. Accessed September 2, 2019.
- ClinicalTrials.gov. A randomized, double-blind, placebo-controlled, multicenter, 16-week treatment study with a 16 week follow-up period to assess the efficacy and safety of dupilumab (anti-il4ra) in adult patients with cholinergic urticaria despite h1-antihistamine treatmen. bethesda (md): national library of medicine. https://clinicaltrials.gov/ct2/show/NCT03749148. Published 2018. Accessed September 2, 2019.
- Moy AP, Murali M, Nazarian RM. Identification of a Th2- and Th17-skewed immune phenotype in chronic urticaria with Th22 reduction dependent on autoimmunity and thyroid disease markers. J Cutan Pathol. 2016;43(4):372- 378. doi:10.1111/cup.12673
- Weller K et al. Total IgE levels are linked to the response of chronic spontaneous urticaria patients to omalizumab. Allergy. 2018;73(12):2406-2408. doi:10.1111/all.13586
- Bachert C, Mannent L, RM N, al et. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: A randomized clinical trial. JAMA. 2016;315(5):469-479. http://dx.doi. org/10.1001/jama.2015.19330.
- Wenzel S et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388(10039):31-44. doi:https://doi.org/10.1016/S0140-6736(16)30307-5






