and extracellular matrix proteins (e.g., laminin and fibronectin).10-12 The anti-microbial, anti-inflammatory, anti-neoplastic, and anti-oxidant effects of onion extract have been well accepted for many years.13 It is also popular in treating postoperative scars.
In this study, we aimed to compare and discuss the immunohistochemical
and ultrastructural effects of Contractubex gel, heparin sodium, and allantoin in the rat model.
METHODS
Study Design
All of the protocols used in the present study were approved by the Animal Research Ethics Committee of Celal Bayar University. Thirty-two female Sprague-Dawley rats, weighing 200 to 230 g were purchased from Experimental Animals Research Laboratory
of Aegean University and used in the study. Guidelines for using of laboratory animals were strictly adhered throughout the study. The rats were fed with standard rodent feed and kept in a 20 °C room. After shaving the hair of the left dorsal side of each rat, an incisional skin biopsy was taken to develop a full-thickness wound. The dimensions of the samples were 1 x 1 x 0.3 cm. The animals were randomly divided into four experimental groups, i.e., a control group (Group 1) and three treatment groups (Groups 2, 3, and 4). After the animals' wounds had completely healed over a period of 10 days, the wounds were treated topically and daily as follows: Group 2 (n=8), extractum cepae-heparin sodium-allantoin
mixture (Contractubex gel, Merz Pharma, Frankfurt, Germany [components: 10% aqueous onion extract, 50 U heparin per gram of gel, 1% allantoin]); Group 3 (n=8), heparin sodium 5000 IU (Liquemine® flac, Roche Pharmaceuticals, Istanbul, Turkey); and Group 4 (n=8), allantoin 1 g (Allantoin, H.G.u.C. Blau GmbH, Hamburg,
Germany). No treatment was applied to control Group 1 (n=8). At the beginning the scars were not keloidal or hypertrophic
in our study. On the 30th day of treatment, scar tissues of the animals in all groups were excised and examined microscopically.
Histochemical and Immunohistochemical Analysis
Specimens were fixed in 10% buffered formaline solution for 24-48 hours and then performed routine paraffin procedure. Sections (5 µm thick) were cut and prepared for both histochemical
and indirect immunohistochemical stainings. For immunohistochemical evaluation, avidin-biotin peroxidase system (Santa Cruz Staining System, immunocruz sc-2051, Santa Cruz, CA) was used. Anti-transforming growth factor beta (anti-TGF-β) (Neomarkers, mouse Mab, MS-1106, Lab Vision
Corp, Fremont, CA), anti-laminin (Neomarkers, rabbit Pab, RB-082, Lab Vision Corp, Fremont, CA), and anti-fibronectin (Neomarkers, mouse Mab, MS-1351, Lab Vision Corp, Fremont, CA), primary antibodies were used. After 18 hr of incubating with primary antibodies at 4 °C, tissue sections were washed with phosphate buffered saline (PBS), then incubated for one hour with streptavidin-peroxidate conjugate (Histostain-DS Kit-95-9999, Zymed Laboratories, San Francisco, CA) in a 1:100 dilution. After a second PBS wash, chromogen diaminobenzidine
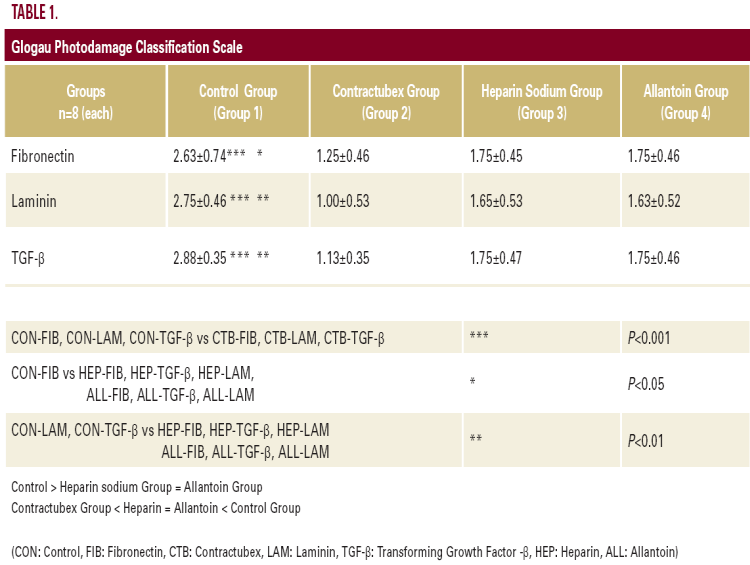
tetrahydrochloride (DAB) was applied to the sections for five minutes for color development, which was followed by counterstaining with Mayer's hematoxylin. A semiquantitative grading system was used to compare the staining intensities using light microscopy. Immunohistochemical staining on each section was graded as mild (+), moderate (++), or strong (+++). ANOVA non-parametric test was used to compare the staining intensities, P<0.05 was accepted as significant (Table 1).






