between Absorica LD and Absorica due to the advanced micronization technology of Absorica LD.
The enhanced absorption level of Absorica LD is based on two clinical trials comparing Absorica LD and Absorica pharmacokinetics. The pharmacokinetics of Absorica LD and Absorica were evaluated in two open-label, randomized, crossover studies in healthy volunteers: the fed bioequivalence and food effect study, and the fasting study.18 In the fed bioequivalence and food-effect study, patients first underwent an overnight fast of ≥10 hours, and then either consumed a high-fat, high-calorie breakfast and were given Absorica 40mg or Absorica LD 32mg, or received no breakfast and were given Absorica LD 32mg. Of note, the high-fat, high-calorie meal used in the study was based on the FDA-stipulated meal and included 2 fried eggs, 2 strips of bacon, 4 oz of hash browns, 2 slices of buttered toast, and 8 oz of whole milk. In the fasting study, patients underwent an overnight fast of ≥10 hours and then received either Absorica 40mg or Absorica LD 32mg. Bioavailability was assessed by measuring area under the plasma concentration-time curve from time 0 to the time of last measurable isotretinoin concentration (AUC0–t), area under the plasma concentration-time curve from time 0 to infinity (AUC0-∞), peak isotretinoin exposure (Cmax), and time to peak isotretinoin exposure (Tmax), in blood samples taken pre-dosing and over 96 hours post-dosing.
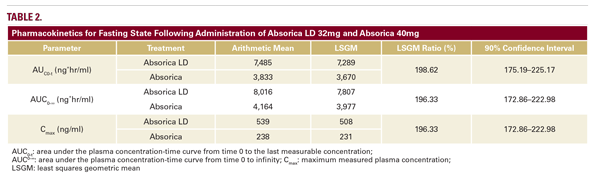
Overall, these studies demonstrated that the absorption of Absorica LD 32mg is superior to Absorica 40mg. The fed bioequivalence and food effect study showed that in under fed conditions, Absorica LD 32mg is bioequivalent to Absorica 40mg. This is demonstrated by the least squares geometric mean (LSGM) ratios for AUC0–t, AUC0-∞, and Cmax for fed-state Absorica LD 32mg versus fed-state Absorica 40mg. These LSGM ratios had 90% confidence intervals (CIs) that all fell within the 80 percent to 125 percent range for bioequivalence (Table 1).18 Absorica LD 32mg, with its micronized technology, achieves similar plasma levels to Absorica 40mg in the fed state with a 20 percent lower dose.18 Furthermore, in the fasted study, Absorica LD 32 mg was absorbed approximately twice as much as Absorica.18 This is evidenced by the AUC0–t, AUC0-∞, and Cmax of fasted-state Absorica LD that were nearly double those for fasted-state Absorica (Table 2).18 Absorica LD 32mg also produces consistent serum levels irrespective of gastrointestinal contents.18 The promising results from these studies suggest that Absorica LD could be an alternative acne therapy without stringent food intake requirements.
Of note, the therapeutic efficacy of Absorica LD is based on a clinical trial comparing Absorica to generic isotretinoin.15,23 A clinical trial has yet to be conducted with Absorica LD. In the phase 3 clinical trial comparing Absorica to generic isotretinoin, Absorica was shown to be non-inferior to

![]()
The enhanced absorption level of Absorica LD is based on two clinical trials comparing Absorica LD and Absorica pharmacokinetics. The pharmacokinetics of Absorica LD and Absorica were evaluated in two open-label, randomized, crossover studies in healthy volunteers: the fed bioequivalence and food effect study, and the fasting study.18 In the fed bioequivalence and food-effect study, patients first underwent an overnight fast of ≥10 hours, and then either consumed a high-fat, high-calorie breakfast and were given Absorica 40mg or Absorica LD 32mg, or received no breakfast and were given Absorica LD 32mg. Of note, the high-fat, high-calorie meal used in the study was based on the FDA-stipulated meal and included 2 fried eggs, 2 strips of bacon, 4 oz of hash browns, 2 slices of buttered toast, and 8 oz of whole milk. In the fasting study, patients underwent an overnight fast of ≥10 hours and then received either Absorica 40mg or Absorica LD 32mg. Bioavailability was assessed by measuring area under the plasma concentration-time curve from time 0 to the time of last measurable isotretinoin concentration (AUC0–t), area under the plasma concentration-time curve from time 0 to infinity (AUC0-∞), peak isotretinoin exposure (Cmax), and time to peak isotretinoin exposure (Tmax), in blood samples taken pre-dosing and over 96 hours post-dosing.
Overall, these studies demonstrated that the absorption of Absorica LD 32mg is superior to Absorica 40mg. The fed bioequivalence and food effect study showed that in under fed conditions, Absorica LD 32mg is bioequivalent to Absorica 40mg. This is demonstrated by the least squares geometric mean (LSGM) ratios for AUC0–t, AUC0-∞, and Cmax for fed-state Absorica LD 32mg versus fed-state Absorica 40mg. These LSGM ratios had 90% confidence intervals (CIs) that all fell within the 80 percent to 125 percent range for bioequivalence (Table 1).18 Absorica LD 32mg, with its micronized technology, achieves similar plasma levels to Absorica 40mg in the fed state with a 20 percent lower dose.18 Furthermore, in the fasted study, Absorica LD 32 mg was absorbed approximately twice as much as Absorica.18 This is evidenced by the AUC0–t, AUC0-∞, and Cmax of fasted-state Absorica LD that were nearly double those for fasted-state Absorica (Table 2).18 Absorica LD 32mg also produces consistent serum levels irrespective of gastrointestinal contents.18 The promising results from these studies suggest that Absorica LD could be an alternative acne therapy without stringent food intake requirements.
Of note, the therapeutic efficacy of Absorica LD is based on a clinical trial comparing Absorica to generic isotretinoin.15,23 A clinical trial has yet to be conducted with Absorica LD. In the phase 3 clinical trial comparing Absorica to generic isotretinoin, Absorica was shown to be non-inferior to








