at baseline (mild effect on QoL) to 0.38 (no effect on QoL) at the EOS/day 84 visit.
DISCUSSION
This study represents the first clinical evaluation of VP-102, a propriety drug-device combination product containing 0.7% cantharidin (w/v), for the topical treatment of MC. While there are no approved treatments for this common skin infection in the US, compounded cantharidin has been used to treat MC and warts for more than 60 years.17 There are several limitations to the use of cantharidin for treatment of MC. Cantharidin’s access is limited due to restriction mandated by federal law, thus requiring physicians to obtain it outside the US or through compounding pharmacies. In addition, there are no data to support an optimized formulation or dosing regimen. Finally, the application and treatment schedule vary by practitioner, are inconsistent in previous studies, and the safety and efficacy of its use in MC has not been proven in large trials.16
The clinical development of VP-102 addresses these issues by seeking FDA approval of a standardized, shelf-stable formulation of cantharidin delivered via a proprietary, single-use applicator. The small tip of the applicator is designed to improve safety and efficacy by targeting MC lesions and sparing surrounding healthy skin, while the gentian violet surgical dye in the solution may assist in reducing duplicative dosing of individual lesions in a single treatment. The single-use applicator may also reduce the potential for cross-contamination, as direct contact with the skin is not necessary for application, and the applicator is not to be used across multiple patients.
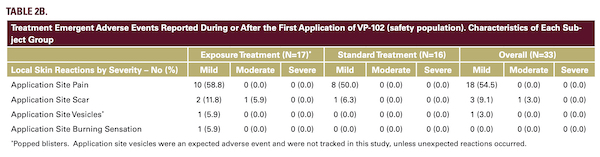
Systemic exposure to cantharidin was negligible in this pediatric patient population, as evidenced by 65/66 plasma samples being below the LLOQ. These findings and the incidence of AEs support the safety of VP-102 to treat MC in the pediatric population. Complete clearance of MC lesions was observed in 48.5% of all VP-102-treated subjects and VP-102 treatment reduced the number of lesions by an average of 90.4% at the EOS/day 84 visit compared to baseline. On the CDLQI, subjects showed an improvement in QoL from a mild effect of disease at baseline to no effect at the end of the study.
The clinical development of VP-102 addresses these issues by seeking FDA approval of a standardized, shelf-stable formulation of cantharidin delivered via a proprietary, single-use applicator. The small tip of the applicator is designed to improve safety and efficacy by targeting MC lesions and sparing surrounding healthy skin, while the gentian violet surgical dye in the solution may assist in reducing duplicative dosing of individual lesions in a single treatment. The single-use applicator may also reduce the potential for cross-contamination, as direct contact with the skin is not necessary for application, and the applicator is not to be used across multiple patients.
Systemic exposure to cantharidin was negligible in this pediatric patient population, as evidenced by 65/66 plasma samples being below the LLOQ. These findings and the incidence of AEs support the safety of VP-102 to treat MC in the pediatric population. Complete clearance of MC lesions was observed in 48.5% of all VP-102-treated subjects and VP-102 treatment reduced the number of lesions by an average of 90.4% at the EOS/day 84 visit compared to baseline. On the CDLQI, subjects showed an improvement in QoL from a mild effect of disease at baseline to no effect at the end of the study.
CONCLUSION
In conclusion, the results of this Phase 2 study suggest that
large randomized clinical trials are warranted to compare topical
VP-102 with a vehicle control in a diverse population of subjects
with MC in order to fully evaluate the safety and efficacy of VP-
102.
DISCLOSURES
The trial and was sponsored by Verrica Pharmaceuticals Inc.
Drafting of the manuscript and creation of the figures were
funded by Verrica Pharmaceuticals. Drs. Niazi, Brabec, and
Anschutz served as clinical study investigators on the trial
and received funding to complete the study. Dr. Davidson, Dr.
Burnett, and Ms. Willson were employees and stockholders
with Verrica Pharmaceuticals Inc. at the time of the study. Dr.
Davidson holds the following patents related to the study:
WO2018226894A1, WO2018232277A1, WO2016100732A3,
WO2016118633A1, WO2015027111A1, W02014308690.
ACKNOWLEDGMENT
We thank the subjects and caregivers who were a part of the
study, as well as Dr. Jessica McLin (Versant Learning Solutions,
LLC) for her assistance with drafting the manuscript and figures,
and Dr. Melissa Olivadoti for her assistance in coordinating author
reviews and editorial changes.
REFERENCES
- Hay RJ, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014; 134:1527- 1534.
- Dohil MA, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54.
- Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infect Dis. 2013;13:877-88.
- Leung AK. The natural history of molluscum contagiosum in children. Lancet Infect Dis. 2015;15:136-137.
- Olsen JR, et al. Quality of life impact of childhood skin conditions measured using the Children's Dermatology Life Quality Index (CDLQI): a metaanalysis. Br J Dermatol. 2016;174:853-861.
- Olsen JR, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195.
- van der Wouden JC, et al. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2017;5:CD004767.
- Silverberg N. Pediatric molluscum contagiosum: optimal treatment strategies. Paediatr Drugs. 2003;5:505-512.
- Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatric Dermatology. 2009;26:405- 408.
- Moye V, et al. Beetle juice: a guide for the use of cantharidin in the treatment of molluscum contagiosum. Dermatologic Therapy. 2013;26:445-451.
- Bertaux B, et al. Cantharide acantholysis: endogenous protease activation leading to desmosomal plaque dissolution. Br J Dermatol. 1988;118:157-165.
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43: 503-507
- Hanna D, et al. A prospective randomized trial comparing the efficacy and adverse effects of four recognized treatments of molluscum contagiosum in children. Pediatr Dermatol. 2006;23:574-579.
- Cathcart S, Coloe J, Morrell DS. Parental satisfaction, efficacy, and adverse events in 54 patients treated with cantharidin for molluscum contagiosum infection. Clin Pediatr. 2009;48:161-165.
- Moye VA, Cathcart S, Morrell DS. Safety of cantharidin: a retrospective review of cantharidin treatment in 405 children with molluscum contagiosum. Pediatr Dermatol. 2014;31:450-454.
- Del Rosso, JQ, Kircik L. Topical cantharidin in the management of molluscum contagiosum: preliminary assessment of an ether-free, pharmaceuticalgrade formulation. J Clin Aesthet Dermatol. 2019;12:27-30.
- Forbat E, Al-Niaimi F, Ali FR. Molluscum contagiosum: review and update on management. Pediatr Dermatol. 2017;34:504-515.
AUTHOR CORRESPONDENCE
Cynthia Willson RN BSN cwillson@verrica.com






