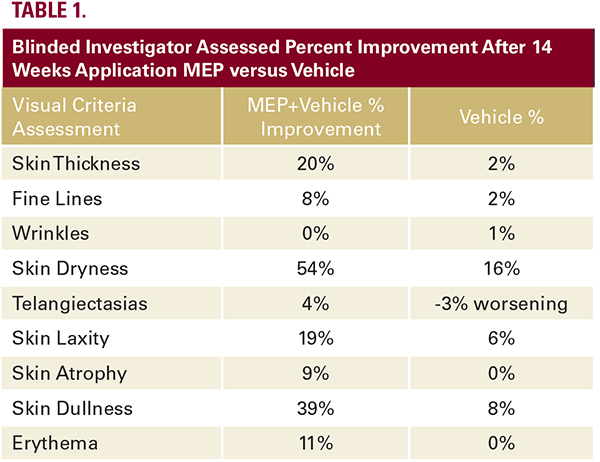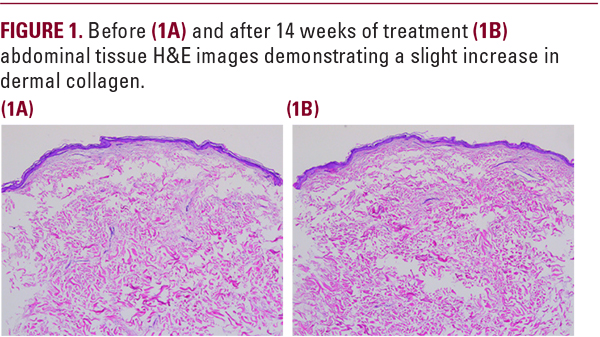
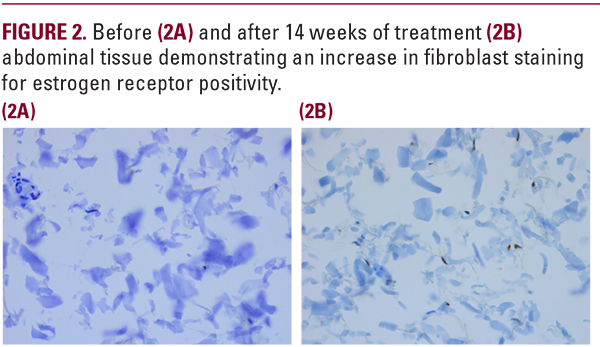
 with the length of time required to obtain statistical signficance related to functional skin improvement as compared to cosmetic moisturizer benefit. The percent change differences between baseline and week 14 for MEP versus vehicle are summarized in Table 1. Five of nine subjects who participated in the biopsy study demonstrated a slight increase in dermal collagen (Figure 1) with four of nine subjects showing an increase in fibroblasts staining positively for estrogen receptors (Figure 2).
with the length of time required to obtain statistical signficance related to functional skin improvement as compared to cosmetic moisturizer benefit. The percent change differences between baseline and week 14 for MEP versus vehicle are summarized in Table 1. Five of nine subjects who participated in the biopsy study demonstrated a slight increase in dermal collagen (Figure 1) with four of nine subjects showing an increase in fibroblasts staining positively for estrogen receptors (Figure 2).DISCUSSION
This research evaluated the safety and efficacy of MEP in topical formulation applied twice daily in postmenopausal females who had not taken HRT. The safety of topical MEP was demonstrated by the lack of active MEP and the presence of the carboxylic acid MEP inactive metabolite in the serum of subjects who has used the formulation for 12 weeks. This soft effect allowed targeted delivery of the MEP to the skin without systemic side effects, necessary for a cosmeceutical. Restoration of estrogen-like skin effects might induce the production of collagen I, responsible for the strength of the skin, and collagen III, contributing to the elastic skin properties, while reducing the expression of matrixmetalloprotease 1 (MMP-1).11 The efficacy study carried out for 14 weeks demonstrated a slight increase in collagen from the abdominal biopsies where the MEP was applied twice daily. An increase in fibroblast expressed estrogen receptors was also seen in some subjects as compared to baseline. A more dramatic effect would likely be observed if the study had been carried out longer.The study was designed to eliminate any moisturizer effect from the vehicle, which can be a confounding variable in cosmeceuticalstudies. The vehicle in many formulations is responsible for the observed benefits and not the active hero ingredient. This was not the case in this research. Only the MEP could have produced the statistically improved week 14 investigator observations seen in dryness, laxity, atrophy, and dullness over vehicle. The improvement in dryness, usually attributed to the vehicle, may have been due to an improved extracellular matrix with enhanced water holding capabilities from hydrophilic glycosaminoglycans.12,13 Improvement over vehicle seen by the investigator in laxity and atrophy is consistent with early estrogen- like collagen induced effects. Finally, reduced dullness might have been due to the estrogen-like effect of MEP on the cutaneous microcirculaton.14 These appearance benefits point to the possible value of MEP as a cosmeceutical ingredient in the treatment of EDS.Procedures exist for addressing skin surface texture (peels, microdermabrasion, and dermabrasion), facial lines (chemodenervation), and folds (hyaluronic acid fillers), but no current procedures exist for improving the quality of facial skin. If the facial skin could be made more robust, it would drape more artistically over the underlying bones providing a better canvas for other facial rejuvenation procedures. Estrogen deficient skin is thin, fragile, and heals poorly.14 Microdroplet injection of hyaluronic acid and the use of microneedling or fractionated laser with active ingredients have attempted to improve skin thickness with limited success. Inducing an estrogen-like effect in the skin through use of a cosmeceutical could represent a noninvasivemethod for improving skin quality and potentially making other cosmetic procedures more effective. Further research is needed with extended subject product use, but MEP appears to be a promising ingredient in estrogen deficient females.