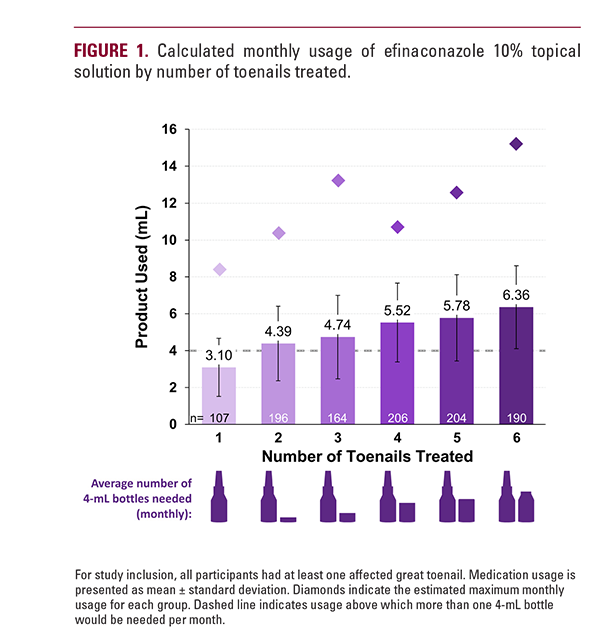


1.17 to 1.32 4 mL bottles monthly (Figure 2). Because application instructions specify that the nail plate, toenail folds, toenail bed, hyponychium, and nail plate undersurface should be completely covered, regardless of the area of involvement, it was expected that medication usage might be similar for nails with different surface areas affected.5
DISCUSSION
In these clinical trials, participants were provided 10 mL of efinaconazole per month. In clinical practice, however, almost 90% of prescriptions for efinaconazole are for one 4 mL bottle monthly.3 For patients with 2 or more affected toenails, a 4 mL bottle would likely be depleted in under a month, and after as few as 19 days with 6 affected nails, leaving treatment gaps until prescriptions are refilled. Because intermittent treatment may affect medication efficacy and increase the likelihood of relapse or reinfection,1 patients with more than one nail involved might be more likely to achieve success with an 8 mL bottle of efinaconazole (Figure 3). Given that nail percent involvement, sex, and BMI do not affect medication usage, number of affected nails should be the major consideration when determining the monthly efinaconazole quantity to prescribe.






