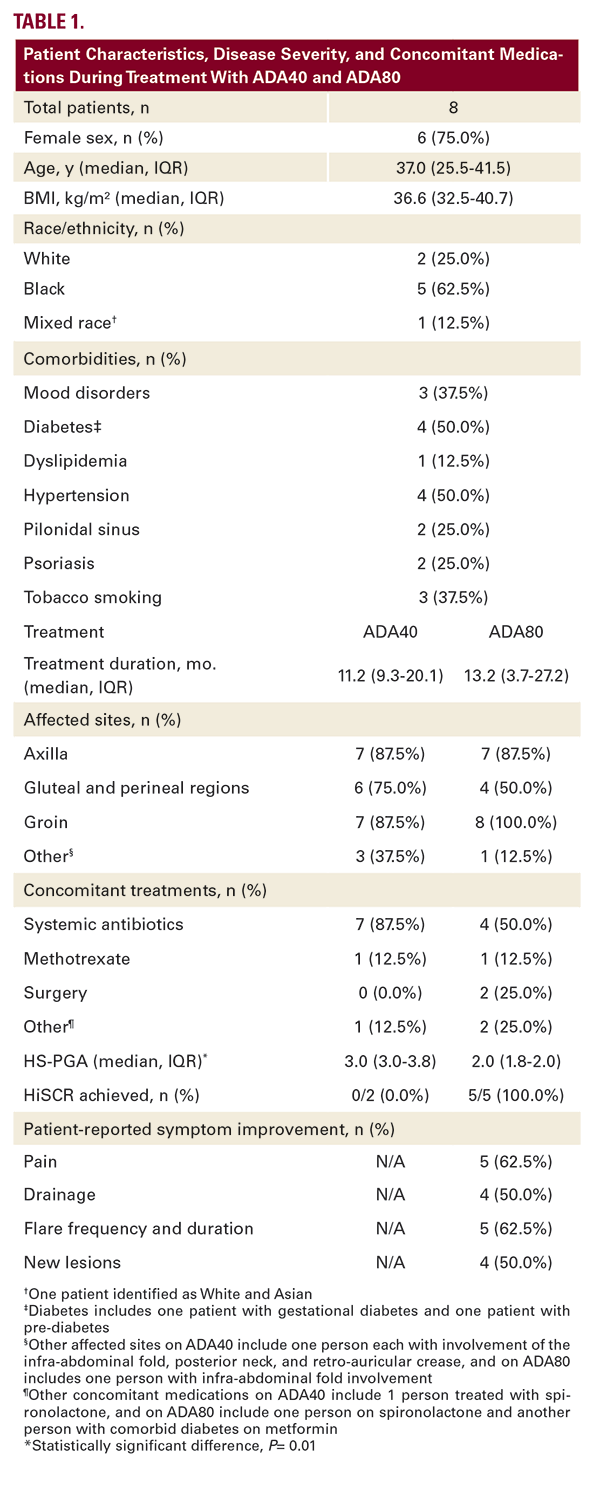
Disease improvement was not observed on ADA40 in any of the 6 patients for whom pre-ADA40 data were available (pre-ADA40: median HS-PGA 3.0 (3.0-3.0); ADA40: HS-PGA 3.0 (3.0-3.8), P=0.08); HiSCR was not achieved in the 2 patients for whom lesion counts were available). At time of dose escalation, all patients had recurrent and new inflammatory lesions, pain, and drainage on ADA40.
On ADA80, patients experienced one-point reduction in median HS-PGA (2.0 (1.8-2.0), P=0.01). HiSCR was achieved in all 5 patients for whom lesion counts were available. All patients reported improvement in HS symptoms, including reduced pain, drainage, lesions, and frequency and duration of flares on ADA80. No adverse events were reported with ADA80.
DISCUSSION
We report improved median HS-PGA and patient-reported symptomatology in response to ADA80 treatment in overweight and obese patients with moderate-to-severe HS who failed to respond to ADA40. It is noteworthy that two patients achieved sufficient disease improvement on ADA80 to become eligible for definitive surgical management.
Obesity is commonly associated with HS. Obese patients who are prescribed TNF-inhibitors for immune-mediated inflammatory diseases are more likely to fail treatment than non-obese patients.7 It is yet to be determined if treatment failure is due to inadequate serum drug concentration or unknown obesity-related factors that make TNF-inhibitors less effective.
Our study contributes to the sparse literature evaluating dose escalation for HS patients with insufficient response to ADA40. In a European study, data from a previously-reported retrospective study of 14 White European patients treated with ADA80 for 12 weeks4 were combined with retrospective data from 8 additional patients treated with ADA80 for 12 weeks and prospective data from 8 patients treated with adalimumab 80 mg every 12 days and 5 patients treated with adalimumab 80 mg every 10 days over a 24-week period, which demonstrated HiSCR-achievement in 66% of patients and median reduction of disease severity from severe to moderate.5 Given that this study compares 3 different dosing intervals in one ethnic group, our findings generated from a racially diverse and more obese (median BMI 36.6 (32.5–40.7) vs 29.0 (25.3–33.4)) American cohort using a single dosing interval for a longer treatment period (13.2 (3.7–27.2) months vs 3–6 months) add meaningful clarity regarding the effectiveness of ADA80 for overweight and obese patients with moderate-to-severe HS.
Our study highlights the limitations of existing HS outcome measures. HS-PGA and HiSCR are responsive to disease change but are based on lesion counts which have been shown to have poor inter-rater reliability.8 Moreover, HiSCR may have limited ability to capture treatment response in disease with extensive






