improving, improving, or worsening, respectively) and physician clinical assessments (complete response, partial response, or non-response, respectively). Qualitative descriptions of cutaneous and functional outcomes were provided through chart notes.
RESULTS
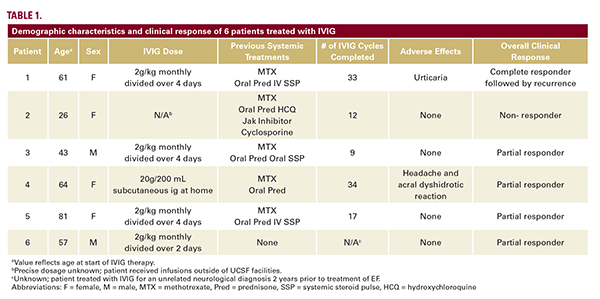
Of 226 patients with morphea, we identified 18 patients (8.0%) with EF, of whom 6 patients (33.3%) had detailed IVIG follow-up data (Table 1). All patients had functional impairments prior to IVIG initiation. IVIG was administered with a corticosteroid and a steroid-sparing agent (SSA) in 5 patients (83.3%); 1 patient (16.7%) received a SSA only. While IVIG was never provided as a monotherapy, 5 patients (83.3%) discontinued oral corticosteroids while on IVIG. The average duration of IVIG therapy was 20.6 +/- 16.4 months, and the standard IVIG dose was 2 g/kg monthly divided over 2-4 days (Table 1).
Four patients (66.7%) reported both cutaneous and functional improvement within 2 months (Table 2). Both cutaneous and functional improvement were sustained across subsequent IVIG cycles in 5 patients (83.3%). Four patients (66.7%) were partial responders (Table 1). One patient (16.7%) achieved complete remission after 36.3 months of therapy; they relapsed with focal truncal involvement within 3 months of discontinuation, but without new functional deficits, and IVIG was not resumed. One patient (16.7%) was a non-responder with new lesions and persistent polyarthralgias who discontinued IVIG and achieved a partial response with mepolizumab. Adverse effects of IVIG included urticaria without systemic symptoms in 1 patient (16.7%) and headaches, malaise, and an acral dyshidrotic reaction in 1 patient (16.7%). No patients experienced a thromboembolic or veno-occlusive event.

DISCUSSION
This study expands on limited available data supporting IVIG as a well-tolerated add-on therapy for patients with EF who suffer persistent cutaneous disease and functional impairment despite corticosteroids and SSAs. Although concomitant treatment with corticosteroids and SSAs may have reduced our ability to isolate the effects of IVIG, we emphasized careful comparison of patient and provider-reported clinical findings before, during, and after treatment courses with and without IVIG to thoroughly characterize its impact. While this small cohort study is limited by its retrospective nature and the rarity of EF, we present






