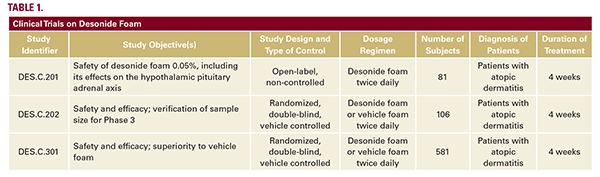
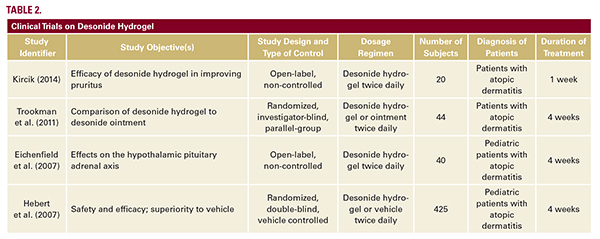
 while minimizing greasy sensations and appearance—may increase patient compliance with therapeutic regimens.Desonide foam was evaluated in three clinical trials of patients with atopic dermatitis (Table 1).20 The efficacy of desonide foam applied twice daily for four weeks was evaluated in a phase III, double-blind, randomized, multicenter trial in patients aged 3 months to 17 years. Clinical success was defined as meeting all of the following criteria: Investigator’s Static Global Assessment (ISGA) score of clear or almost clear, a minimum two-grade improvement in the ISGA scores from baseline, and absent or minimal erythema and induration/papulation. Of the 387 subjects treated with desonide, 39% achieved clinical success versus 9% in the vehicle group (P less than 0.0001). Individual primary endpoint results indicated that 41% of subjects achieved an ISGA of clear or almost clear (versus 9% on vehicle), 68% of subjects achieved absent or minimal erythema (versus 36% on vehicle), and 69% of subjects achieved absent or minimal induration/papulation (versus 38% on vehicle; P less than 0.0001).18, 20 The safety profile of desonide foam is consistent with that of preceding formulations. Combined safety data from phase II and III studies (768 subjects in total, 540 receiving desonide) revealed that 6% of subjects receiving desonide experienced adverse events, as compared to 14% of subjects receiving vehicle foam (P equals 0.0002).18, 20, 21 The majority of these adverse events were local skin irritations that were transient in nature, mild-to-moderate in severity, and independent of age, race, or gender. As HPA axis suppression is an adverse event of special concern in pediatric patients, the effect of desonide 0.05% foam on the HPA axis was evaluated in a 4-week phase II, multicenter, open-label study of adolescent and pediatric participants with mild-to-moderate AD. Of 75 participants, three subjects (4%) experienced mild, transient HPA axis suppression as determined by postcosyntropin stimulation serum cortisol levels. There was no increase in the risk of HPA axis suppression for infants and younger children compared with adolescents.20 Shortly after the approval of desonide foam, a hydrogel formulation was also approved in 2006. Several trials have since demonstrated the safety and efficacy of desonide hydrogel in atopic dermatitis (Table 2).15, 22-24 In particular, desonide hydrogel has been shown to be equally
while minimizing greasy sensations and appearance—may increase patient compliance with therapeutic regimens.Desonide foam was evaluated in three clinical trials of patients with atopic dermatitis (Table 1).20 The efficacy of desonide foam applied twice daily for four weeks was evaluated in a phase III, double-blind, randomized, multicenter trial in patients aged 3 months to 17 years. Clinical success was defined as meeting all of the following criteria: Investigator’s Static Global Assessment (ISGA) score of clear or almost clear, a minimum two-grade improvement in the ISGA scores from baseline, and absent or minimal erythema and induration/papulation. Of the 387 subjects treated with desonide, 39% achieved clinical success versus 9% in the vehicle group (P less than 0.0001). Individual primary endpoint results indicated that 41% of subjects achieved an ISGA of clear or almost clear (versus 9% on vehicle), 68% of subjects achieved absent or minimal erythema (versus 36% on vehicle), and 69% of subjects achieved absent or minimal induration/papulation (versus 38% on vehicle; P less than 0.0001).18, 20 The safety profile of desonide foam is consistent with that of preceding formulations. Combined safety data from phase II and III studies (768 subjects in total, 540 receiving desonide) revealed that 6% of subjects receiving desonide experienced adverse events, as compared to 14% of subjects receiving vehicle foam (P equals 0.0002).18, 20, 21 The majority of these adverse events were local skin irritations that were transient in nature, mild-to-moderate in severity, and independent of age, race, or gender. As HPA axis suppression is an adverse event of special concern in pediatric patients, the effect of desonide 0.05% foam on the HPA axis was evaluated in a 4-week phase II, multicenter, open-label study of adolescent and pediatric participants with mild-to-moderate AD. Of 75 participants, three subjects (4%) experienced mild, transient HPA axis suppression as determined by postcosyntropin stimulation serum cortisol levels. There was no increase in the risk of HPA axis suppression for infants and younger children compared with adolescents.20 Shortly after the approval of desonide foam, a hydrogel formulation was also approved in 2006. Several trials have since demonstrated the safety and efficacy of desonide hydrogel in atopic dermatitis (Table 2).15, 22-24 In particular, desonide hydrogel has been shown to be equally





