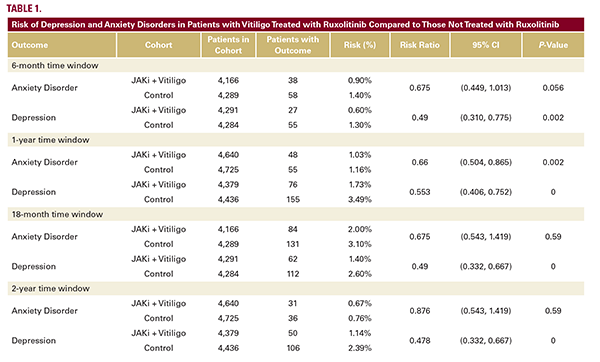
To the Editor,
Vitiligo, a chronic autoimmune condition characterized by loss of melanocytes and resultant skin depigmentation, is associated with reduced quality of life, and increased risks of psychiatric comorbidities such as depression and anxiety.1-3 While current clinical assessments focus on improving objective markers of disease burden, such as percentage of Body Surface Area (BSA), little is known about the effect of vitiligo treatment on other patient outcomes like depression and anxiety.
Ruxolitinib, a topical Janus kinase (JAK) inhibitor, is a novel and effective therapy approved for repigmentation in nonsegmental vitiligo.3 Despite its growing use, its potential impact on depression and anxiety rates in treated patients has not yet been reported.
In this retrospective cohort study, we used the TriNetX Network to examine rates of depression and anxiety in vitiligo patients treated with ruxolitinib. The TriNetX Network allows examination of claims and electronic health record data for patients from 24 US healthcare organizations. This type of claims-level analysis offers, among its advantages, the ability to evaluate large numbers of patients, to associate different clinical states in each patient (via ICD codes), and to evaluate interventions via pharmacy claims data.
Vitiligo, a chronic autoimmune condition characterized by loss of melanocytes and resultant skin depigmentation, is associated with reduced quality of life, and increased risks of psychiatric comorbidities such as depression and anxiety.1-3 While current clinical assessments focus on improving objective markers of disease burden, such as percentage of Body Surface Area (BSA), little is known about the effect of vitiligo treatment on other patient outcomes like depression and anxiety.
Ruxolitinib, a topical Janus kinase (JAK) inhibitor, is a novel and effective therapy approved for repigmentation in nonsegmental vitiligo.3 Despite its growing use, its potential impact on depression and anxiety rates in treated patients has not yet been reported.
In this retrospective cohort study, we used the TriNetX Network to examine rates of depression and anxiety in vitiligo patients treated with ruxolitinib. The TriNetX Network allows examination of claims and electronic health record data for patients from 24 US healthcare organizations. This type of claims-level analysis offers, among its advantages, the ability to evaluate large numbers of patients, to associate different clinical states in each patient (via ICD codes), and to evaluate interventions via pharmacy claims data.