INTRODUCTION
Palmoplantar pustulosis (PPP) is a chronic, debilitating autoimmune skin disease characterized by the presence of recurrent neutrophilic pustules on acral surfaces. Although similarities exist, PPP is currently regarded as a distinct pathologic entity from palmoplantar psoriasis.1 PPP is often refractory to topical treatments and therefore may benefit from the introduction of systemic or biologic therapy, although options are limited at this time. Few, yet promising, case reports exist highlighting the efficacy of oral apremilast, a phosphodiesterase-4 (PDE-4) inhibitor. Herein, we present a case of biopsy-proven PPP successfully treated with apremilast, along with a brief review of relevant literature supporting the use of apremilast.
CASE REPORT
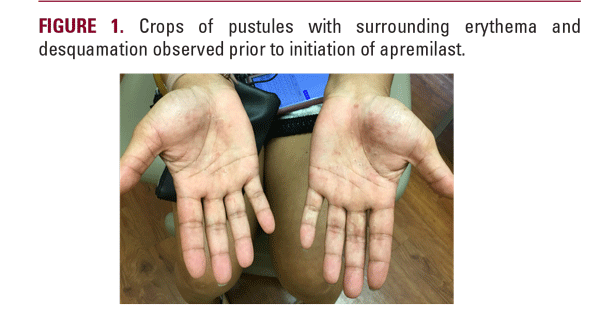
A 39-year-old African American female with a 10 pack-year smoking history presented to the dermatology clinic with a 6-month history of pruritic skin lesions on her hands. Physical examination demonstrated crops of pustules with associated erythema and desquamation located on the palmar surface bilaterally (Figure 1); punch biopsy revealed subcorneal pustules with neutrophils and parakeratosis, consistent with a diagnosis of palmoplantar pustulosis.
She was initially treated with topical corticosteroids in the form of triamcinolone acetonide ointment, followed by halobetasol ointment, with little to no relief. The decision was ultimately made to initiate systemic therapy with apremilast. Nearcomplete resolution of the lesions was observed following 8 weeks of treatment (Figure 1), with significant improvement in associated pruritis and overall quality of life according to the patient. Disease remission was maintained after 6 months, and the medication was well-tolerated by the patient without any reported side effects.
She was initially treated with topical corticosteroids in the form of triamcinolone acetonide ointment, followed by halobetasol ointment, with little to no relief. The decision was ultimately made to initiate systemic therapy with apremilast. Nearcomplete resolution of the lesions was observed following 8 weeks of treatment (Figure 1), with significant improvement in associated pruritis and overall quality of life according to the patient. Disease remission was maintained after 6 months, and the medication was well-tolerated by the patient without any reported side effects.
DISCUSSION
Apremilast works via PDE-4 inhibition, resulting in an upregulation of intracellular cyclic adenosine monophosphate (cAMP) and subsequent suppression of interleukin-2 (IL- 2), interleukin-8 (IL-8), interferon gamma (IFN-γ), and tumor necrosis factor-alpha (TNF-α).2,3 Furthermore, increased levels of IL-8 have been demonstrated in lesions of PPP. Given its neutrophil chemoattractant properties, IL-8 is implicated in the underlying development of PPP.1 The efficacy of apremilast in PPP is therefore presumed to be an effect of IL-8 inhibition, thus preventing formation of the characteristic pustules.
PPP refractory to topical treatments may benefit from the introduction of systemic and/or biologic agents. Anecdotal reports of success with etanercept (TNF-α inhibition), ustekinumab (IL-12/23 inhibitions), and secukinumab (IL-17 inhibition) exist, however, evidence is lacking and inconclusive.4-6 Guselkumab, an IL-23 inhibitor, has demonstrated efficacy in a randomized controlled trial (RCT) and is currently approved for PPP in Japan.7
Systemic agents including methotrexate, acitretin, and cyclosporine have all shown efficacy, however, the adverse side effects associated with the aforementioned agents makes their use disfavorable.8 Side effects of apremilast, on the other hand, primarily consist of diarrhea
and depression, which are typically

PPP refractory to topical treatments may benefit from the introduction of systemic and/or biologic agents. Anecdotal reports of success with etanercept (TNF-α inhibition), ustekinumab (IL-12/23 inhibitions), and secukinumab (IL-17 inhibition) exist, however, evidence is lacking and inconclusive.4-6 Guselkumab, an IL-23 inhibitor, has demonstrated efficacy in a randomized controlled trial (RCT) and is currently approved for PPP in Japan.7
Systemic agents including methotrexate, acitretin, and cyclosporine have all shown efficacy, however, the adverse side effects associated with the aforementioned agents makes their use disfavorable.8 Side effects of apremilast, on the other hand, primarily consist of diarrhea
and depression, which are typically








