INTRODUCTION
Atopic dermatitis (AD) is a common inflammatory skin disease that can significantly affect a patient's quality of life. The pathogenesis of AD is multifactorial and is associated with the dysregulation of type 2 helper T cells and increased production of interleukins 4, 13, and 31.1 A recent study found that the global 1-year period AD prevalence rate and affected population were estimated to be 2.6% and 204.05 million people, respectively.2 Common treatments for this condition include topical steroids, emollients, calcineurin inhibitors, narrowband UVB phototherapy, methotrexate, cyclosporine, biologic therapies, and Janus kinase inhibitors (JAKi), among others.1,3 While combination biologic therapy with dupilumab and various JAKi has been utilized previously, we report a unique case of a patient treated with lebrikizumab in combination with upadacitinib after unsuccessful treatment with upadacitinib 30 mg monotherapy.
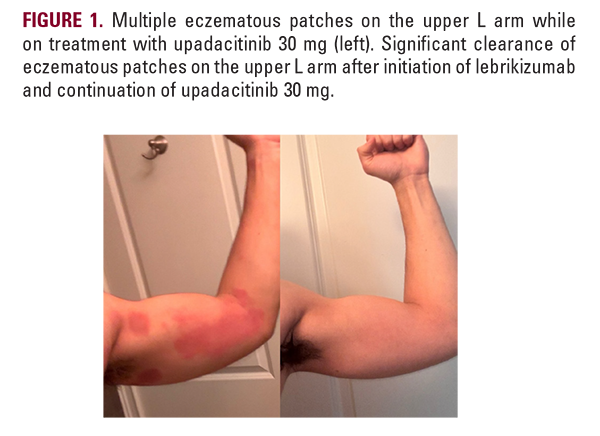
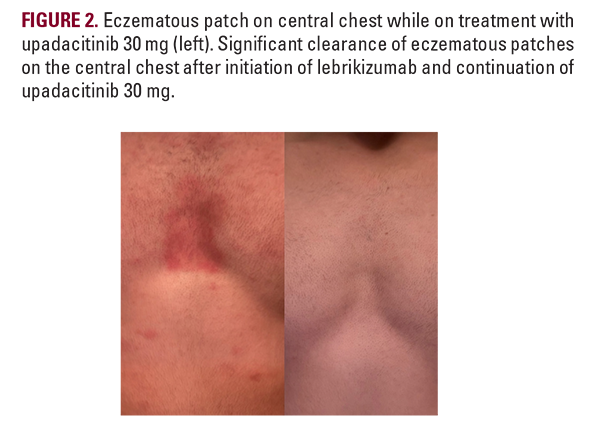
An 18-year-old male presented to our clinic with a two-year history of well-demarcated eczematous plaques throughout the chest, neck, and lateral arms. At the time, he was treated with dupilumab 300 mg injections every two weeks and desoximetasone cream as needed without adequate treatment response. After initiating upadacitinib 15 mg for one month, he experienced mild improvement of the eczematous plaques and was then started on upadacitinib 30 mg. While on upadacitinib 30 mg, the patient reported gradual clearing of his eczematous plaques throughout the chest, neck, and arms. Six months later, he experienced an AD flare resulting in eczematous patches throughout the trunk, upper extremities, and periorbital area that did not resolve with the upadacitinib 30 mg maintenance dose (Figures 1 and 2). His presentation was clinically consistent with an AD flare, and fungal cultures were taken from the chest and axilla to evaluate for tinea corporis, which were negative. Lebrikizumab was added at a loading dose of 500 mg at weeks 0 and 2 followed by the maintenance dose of 250 mg injection every two weeks. While on the combined regimen of upadacitinib and lebrikizumab, he reported significant improvement and clearance of the eczematous patches throughout his body. He continues to remain clear on the combination maintenance dose of lebrikizumab 250 mg and upadacitinib 30 mg (Figures 1 and 2).
An 18-year-old male presented to our clinic with a two-year history of well-demarcated eczematous plaques throughout the chest, neck, and lateral arms. At the time, he was treated with dupilumab 300 mg injections every two weeks and desoximetasone cream as needed without adequate treatment response. After initiating upadacitinib 15 mg for one month, he experienced mild improvement of the eczematous plaques and was then started on upadacitinib 30 mg. While on upadacitinib 30 mg, the patient reported gradual clearing of his eczematous plaques throughout the chest, neck, and arms. Six months later, he experienced an AD flare resulting in eczematous patches throughout the trunk, upper extremities, and periorbital area that did not resolve with the upadacitinib 30 mg maintenance dose (Figures 1 and 2). His presentation was clinically consistent with an AD flare, and fungal cultures were taken from the chest and axilla to evaluate for tinea corporis, which were negative. Lebrikizumab was added at a loading dose of 500 mg at weeks 0 and 2 followed by the maintenance dose of 250 mg injection every two weeks. While on the combined regimen of upadacitinib and lebrikizumab, he reported significant improvement and clearance of the eczematous patches throughout his body. He continues to remain clear on the combination maintenance dose of lebrikizumab 250 mg and upadacitinib 30 mg (Figures 1 and 2).
As standalone therapies for atopic dermatitis, lebrikizumab, and upadacitinib demonstrate robust treatment responses against AD. Lebrikizumab is an IgG4 monoclonal antibody that targets and binds interleukin-13, a key cytokine involved in the pathogenesis of AD.1 The ADvocate1 and ADvocate 2 trials demonstrated a significantly greater number of lebrikizumab-treated patients achieving an IGA score of 0 or 1 with a reduction






