INTRODUCTION
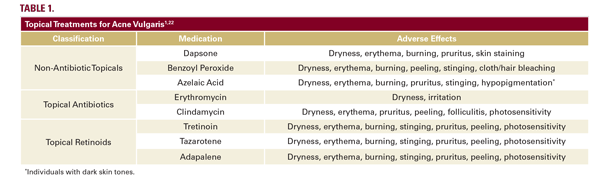
The pathogenesis of acne vulgaris (AV) is multifactorial, and treatments are used in combination to address the multiple pathogenic mechanisms and concurrent non-inflammatory and inflammatory lesions.1,2 Topical retinoids and benzoyl peroxide are among the first line agents (Table 1; Table 2).4,6 Poor adherence to acne treatment is common and is exacerbated by the complexity of treatment regimens.
A combination product, tretinoin 0.1% and benzoyl peroxide 3% (Tret-BPO) cream is approved by the Food and Drug Administration (FDA) for the treatment of AV in patients 9 years of age and older.3 Benzoyl peroxide can oxidize and degrade tretinoin, depending on laboratory conditions, including the presence of light.4,6,7 The Tret-BPO cream uses an encapsulation system that may enhance the stability of both tretinoin and benzoyl peroxide.7
This narrative review will focus on the mechanism of action, pharmacokinetics, efficacy, and safety of the Tret-BPO combination drug in the treatment of AV.
A combination product, tretinoin 0.1% and benzoyl peroxide 3% (Tret-BPO) cream is approved by the Food and Drug Administration (FDA) for the treatment of AV in patients 9 years of age and older.3 Benzoyl peroxide can oxidize and degrade tretinoin, depending on laboratory conditions, including the presence of light.4,6,7 The Tret-BPO cream uses an encapsulation system that may enhance the stability of both tretinoin and benzoyl peroxide.7
This narrative review will focus on the mechanism of action, pharmacokinetics, efficacy, and safety of the Tret-BPO combination drug in the treatment of AV.







