INTRODUCTION
In this retrospective study of patients affected by plaque psoriasis who underwent tildrakizumab therapy, the aim was to describe and compare the response of the nail psoriasis and the plaque psoriasis elsewhere in the body. Clinical charts were reviewed for: demographics, psoriasis severity (Psoriasis Area Severity Index [PASI], mNAPSI [modified Nail Psoriasis Area Severity Index], F-PGA [fingernail physician global assessment]), disease duration, and previous therapies.
Eight patients treated with tildrakizumab - 4 males and 4 females with a mean age of 61 years affected by psoriasis (mean baseline- PASI:13) with nail involvement (mean baseline mNAPSI: 51.9) - were followed for at least 20 weeks. Demographics, disease duration, previous therapies, and severity indexes are reported in Table 1. Four patients were naïve to biological therapies.
At week 4, the mean PASI was 6.6 (49% improvement), and the mean mNAPSI was 30.8 (40.6% improvement). At week 20, the
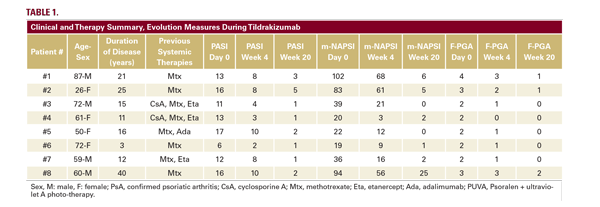
Eight patients treated with tildrakizumab - 4 males and 4 females with a mean age of 61 years affected by psoriasis (mean baseline- PASI:13) with nail involvement (mean baseline mNAPSI: 51.9) - were followed for at least 20 weeks. Demographics, disease duration, previous therapies, and severity indexes are reported in Table 1. Four patients were naïve to biological therapies.
At week 4, the mean PASI was 6.6 (49% improvement), and the mean mNAPSI was 30.8 (40.6% improvement). At week 20, the







