INTRODUCTION
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease characterized by painful nodules, abscesses, and sinus tracts. Beyond cutaneous symptoms, HS is associated with increased risk of metabolic and cardiovascular comorbidities such as obesity, dyslipidemia, metabolic syndrome, and type 2 diabetes mellitus (T2DM).1 Guideline-approved biologics for moderate-to-severe HS include the TNF-α inhibitor adalimumab and IL-17 inhibitors secukinumab and bimekizumab.2 While effective for skin disease, their impact on T2DM and other cardiometabolic comorbidities in HS patients remains largely unexplored in large cohort studies.
Using the TriNetX Research Network (>130 million patients across 80 US healthcare organizations), adults greater than or equal to 18 years with HS were identified by greater than or equal to 2 ICD-10 diagnoses (L73.2) at least one month apart. Patients treated with adalimumab, secukinumab, or bimekizumab were categorized as the biologic cohort and matched 1:1 for age, sex, and race to patients not treated with biologics. The primary outcome was incident T2DM after HS diagnosis. Secondary outcomes included cardiovascular events of myocardial infarction, stroke, and heart failure, in addition to healthcare utilization (emergency department [ED] visits and hospitalizations). A secondary analysis compared these same outcomes between TNF-α and IL-17 inhibitor users. Patients with outcomes prior to HS diagnosis were excluded from the analyses.
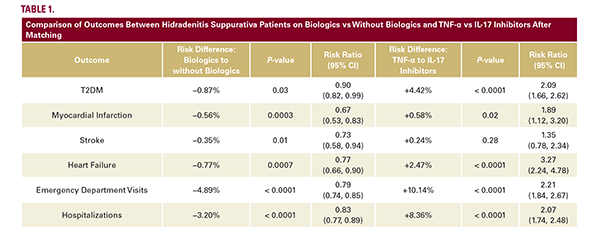
Out of 291,283 patients with HS, 11,669 patients were treated with biologics and 104,076 were not. In the biologic group compared to no biologic use, the mean age was 37.1 vs 36.9 years, respectively, both with over 70% female predominance. 11,669 patients were matched in each cohort. Patients receiving biologic therapy had a significantly (P<0.05) lower risk of developing T2DM, myocardial infarction, stroke, and heart failure compared to matched HS patients not treated with biologics. ED visits and hospitalizations were also reduced. When comparing biologic classes, 10,294 patients were treated with adalimumab (TNF-α inhibitor) and 3,336 with secukinumab or bimekizumab (IL-17 inhibitors). In the TNF-α group compared with IL-17 inhibitors, the mean age was 36.7 vs 39.0 years, respectively, both with over 70% female predominance. 3,336 patients were matched in each cohort. HS patients treated with IL-17 inhibitors had a significantly (P<0.05) lower risk of developing T2DM, myocardial infarction, and heart failure, compared to patients on TNF-α inhibitors. ED visits and hospitalizations were also reduced in the IL-17 inhibitor cohort (Table 1).
Using the TriNetX Research Network (>130 million patients across 80 US healthcare organizations), adults greater than or equal to 18 years with HS were identified by greater than or equal to 2 ICD-10 diagnoses (L73.2) at least one month apart. Patients treated with adalimumab, secukinumab, or bimekizumab were categorized as the biologic cohort and matched 1:1 for age, sex, and race to patients not treated with biologics. The primary outcome was incident T2DM after HS diagnosis. Secondary outcomes included cardiovascular events of myocardial infarction, stroke, and heart failure, in addition to healthcare utilization (emergency department [ED] visits and hospitalizations). A secondary analysis compared these same outcomes between TNF-α and IL-17 inhibitor users. Patients with outcomes prior to HS diagnosis were excluded from the analyses.
Out of 291,283 patients with HS, 11,669 patients were treated with biologics and 104,076 were not. In the biologic group compared to no biologic use, the mean age was 37.1 vs 36.9 years, respectively, both with over 70% female predominance. 11,669 patients were matched in each cohort. Patients receiving biologic therapy had a significantly (P<0.05) lower risk of developing T2DM, myocardial infarction, stroke, and heart failure compared to matched HS patients not treated with biologics. ED visits and hospitalizations were also reduced. When comparing biologic classes, 10,294 patients were treated with adalimumab (TNF-α inhibitor) and 3,336 with secukinumab or bimekizumab (IL-17 inhibitors). In the TNF-α group compared with IL-17 inhibitors, the mean age was 36.7 vs 39.0 years, respectively, both with over 70% female predominance. 3,336 patients were matched in each cohort. HS patients treated with IL-17 inhibitors had a significantly (P<0.05) lower risk of developing T2DM, myocardial infarction, and heart failure, compared to patients on TNF-α inhibitors. ED visits and hospitalizations were also reduced in the IL-17 inhibitor cohort (Table 1).