INTRODUCTION
Immune checkpoint inhibitors (ICIs) are a form of immunotherapy that have contributed to substantial improvements in both the morbidity and mortality of melanoma and include monoclonal antibodies targeting cytotoxic T-lymphocyte associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and lymphocyte-activation gene 3 (LAG-3).1 However, they are frequently associated with toxicities, known as immune-related adverse events (irAEs), that can have significant morbidity and be lethal in some cases. The most commonly reported irAEs include cutaneous, endocrine, and gastrointestinal irAEs.2 Specific cutaneous irAEs (irCAEs) include conditions such as maculopapular rash, bullous pemphigoid eruptions, vitiligo-like skin hypopigmentation, and psoriasiform rash.3 While many studies have characterized both irCAEs and systemic irAEs of ICIs separately, few studies have examined the relationship nor association between cutaneous adverse events and systemic adverse events.
As systemic and cutaneous symptoms often overlap, it is important to characterize the relationship and association between them. Furthermore, cutaneous symptoms are often manifestations of underlying systemic disease processes. Thus, understanding this relationship in the context of immunotherapy will be helpful in the management and treatment of these cutaneous adverse reactions.
In this study, we aimed to compare systemic irAEs in melanoma patients taking ICIs, with and without irCAEs. We hypothesized that patients with melanoma treated with ICIs who have irCAEs will have more systemic AEs when compared with individuals who do not have irCAEs. Secondary objectives included comparing patients with and without cutaneous AEs and determining what factors may be associated with the development of cutaneous AEs (ie, presence of concurrent autoimmune disease, histologic subtype of melanoma, melanoma stage, Breslow depth). We also compared the overall survival of patients with and without irCAEs.
MATERIALS AND METHODS
After approval by the Institutional Review Board, we conducted a retrospective chart review of 147 patients ages 18 or older with advanced stage III and stage IV melanoma receiving ICIs including anti-PD-1 (nivolumab and pembrolizumab), anti-CTLA-4 (ipilimumab), and anti-PD-1/LAG-3 (nivolumab/ relatlimab) at Moffitt Cancer Center from 2015 to 2023. Data collection included patient demographics, tumor pathology, ICI type, irAEs diagnoses, and clinical outcomes. Patients who underwent first-line ICI therapy for melanoma constituted the adjuvant group. Those who experienced progression on firstline ICI therapy and subsequently received further ICI therapy were classified under the metastatic group.
The dichotomous variable of interest, 'Presence of Cutaneous adverse events' was assessed with the categorical covariates using the chi-square test and continuous variables using a nonparametric Mann-Whitney U test. The analysis was conducted using SPSS version 29. The Kaplan-Meier survival analysis was conducted using MedCalc version 22. A significance level of 0.05 was used for all tests.
RESULTS
Our cohort consisted of 147 patients, 55 (37.4%) females 98 (66.7%) patients at stage III, and 49 (33.3%) patients at stage IV. Patient characteristics are shown in Table 1. Among the 147 patients who received adjuvant ICI therapy, 71 patients (48.3%) experienced a systemic irAE. Systemic irAE types included gastrointestinal, endocrine, musculoskeletal, hematologic, hepatobiliary, pulmonary, HEENT, infusionrelated reaction, renal, neurologic, and cardiovascular. The most common systemic irAE encountered on adjuvant therapy was gastrointestinal, affecting 30 patients (20.43%), followed by endocrine in 20 patients (13.6%), and musculoskeletal in 19 patients (12.9%; Table 2). A total of 52 patients experienced disease progression on first-line ICI therapy and began metastatic


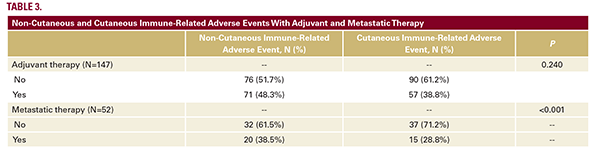
ICI therapy. Of these 52 patients, 20 (38.5%) encountered a systemic irAE. The most prevalent systemic irAE in this cohort was gastrointestinal, affecting 11 patients (10.3%), followed by endocrine in 7 patients (6.5%), and musculoskeletal in 3 patients (2.8%; Table 3).
Fifty-seven (38.8%) patients developed irCAEs with adjuvant therapy. For the patients with an irCAE, the average time in days between initiation of adjuvant therapy and first irCAE was 93.56 days (range: 7 - 524) (N = 55 after excluding two patients for missing data). Among the patients with an irCAE, 54.4% (n=31) also had a systemic irAE. Within this group, the average duration in days from the initiation of adjuvant therapy to the onset of the first irCAE was 86.35 days, while the emergence of a systemic irAE occurred in an average of 109.35 days (range: 1 - 684).
Among the 90 patients without an irCAE, 44.4% had a systemic irAE. There was no significant association between irCAEs and systemic irAEs in the adjuvant treatment group (P=0.240). Of the 52 patients who progressed on first-line adjuvant ICI therapy, 15 (28.8%) patients developed irCAEs while on treatment in the metastatic setting. Among the patients with an irCAE, 53.3% also had systemic irAEs. There was a significant association between irCAEs and systemic irAE in the metastatic treatment group (P<0.001). Among the 37 patients without an irCAE, 32.4% had a systemic irAE.
Among the 57 patients with irCAEs on adjuvant therapy, the most common descriptions of the irCAEs were the following: 32 with a rash (with either pruritic, erythematous, or maculopapular features), 12 with isolated pruritus, 5 with vitiligo, 2 with mucositis, and 1 each with cellulitis, lichen sclerosus, bullous pemphigoid, eczematous reaction, psoriasiform reaction, or urticarial eruptions. Among the 15 patients with irCAEs on metastatic therapy, the most common descriptions of those irCAEs were 6 with a rash (some with pruritus), 3 with isolated pruritus, 2 with vitiligo, 3 with eczematous, and 1 with acneiform eruptions.
The median overall survival for patients who encountered an irCAE was 118 months, compared to 69 months for those who did not experience an irCAE (P=0.102; Figure 1).

DISCUSSION
There was no significant correlation between the incidence of irCAEs and systemic irAEs in the adjuvant group; however, there was a significant correlation in the metastatic treatment group. Only 15 patients in the metastatic group had irCAEs, therefore the small sample may result in clinical insignificance. Though the median survival for patients who developed an irCAE was longer than for patients who did not develop an irCAE, the difference was not statistically significant, likely due to the insufficient sample size. These results are in contrast to several studies that suggest that the development of irCAEs is strongly associated with response to ICI therapy and improved survival.4-6
Limitations of this study include the retrospective design at a single institution. Additionally, there was a lack of histopathology reports for irCAEs, resulting in ambiguity in identifying the specific types of these events, as diagnoses were based on clinical evaluation. Confirmation through histopathology examination could not be determined. Furthermore, the small sample size may restrict meaningful conclusions.
This study highlighted that clinical descriptions of irCAEs are largely vague and refer to irCAEs frequently as diffuse rashes, occasionally adding modifiers such as erythematous, pruritic, and maculopapular. These non-specific descriptions and lack of skin biopsies made it difficult to further characterize irCAEs into more specific sub-groups. We encourage providers seeing patients with irCAEs to describe these reactions in more detail with proper dermatologic terminology, and skin biopsies if necessary for severe cases or those resistant to treatment with topical corticosteroids. Having specific descriptions and histopathologic information is crucial to further characterize the types of irCAEs that exist, understanding the underlying mechanisms of these reactions, and identifying potential alternative treatments.
CONCLUSION
Patients with stage III and stage IV melanoma who progress on
first-line ICI therapy and experience an irCAE are more likely
to also experience a systemic irAE. Additional multi-center
studies are needed to assess the association of irCAE and
systemic irAE in the adjuvant setting. Moreover, understanding
this association may lead to better patient stratification and
personalized treatment approaches, potentially improving the
therapeutic indices of immune checkpoint inhibitors.
DISCLOSURES
The authors have no conflicts of interest to disclose.
REFERENCES
- Paulson KG, Lahman MC, Chapuis AG, Brownell I. Immunotherapy for skin cancer. Int Immunol. 2019;31(7):465-475. doi:10.1093/intimm/dxz012
- Thompson LL, Krasnow NA, Chang MS, et al. Patterns of cutaneous and noncutaneous immune-related adverse events among patients with advanced cancer. JAMA Dermatol. 2021;157(5):577-582. doi:10.1001/ jamadermatol.2021.0326
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83(5):1255- 1268. doi:10.1016/j.jaad.2020.03.132
- Tang K, Seo J, Tiu BC, et al. Association of cutaneous immune-related adverse events with increased survival in patients treated with anti-programmed cell death 1 and anti-programmed cell death ligand 1 therapy. JAMA Dermatol. 2022;158(2):189-193. doi:10.1001/jamadermatol.2021.5476
- Zhang S, Tang K, Wan G, et al. Cutaneous immune-related adverse events are associated with longer overall survival in advanced cancer patients on immune checkpoint inhibitors: A multi-institutional cohort study. J Am Acad Dermatol. 2023;88(5):1024-1032. doi:10.1016/j.jaad.2022.12.048
- Cho YT, Lin YT, Yang CW, et al. Cutaneous immune-related adverse events among Taiwanese cancer patients receiving immune checkpoint inhibitors link to a survival benefit. Sci Rep. 2022;12(1):7021. doi:10.1038/s41598-022- 11128-5
AUTHOR CORRESPONDENCE
Shaliz Aflatooni BS aflatooni@usf.edu






