INTRODUCTION
The purpose of this Supplement is to provide a brief overview of retrometabolic drug design, summarize the impact of the retrometabolic approach in other specialties, and highlight the use of retrometabolically designed drugs under investigation in dermatology, and specifically for hyperhidrosis.
Brief Overview of Retrometabolic Drug Design
Pioneered by Dr. Nicholas Bodor and colleagues in the 1970s, the concept of retrometabolic drug design is defined as the development of an active compound that is readily and rapidly metabolized to inactive moieties or contains designed-in sequential metabolic activity leading to rapid metabolism after performing desired local pharmacological activity. This approach is based on the interaction of structure-function, structure-activity, and structure-metabolic relationships.1 Retrometabolic drug design is characterized by metabolically sensitive moieties that promote rapid metabolism upon entry into the bloodstream and organs, thus reducing unwanted side effects.1 Traditional drug design can increase pharmacological activity, but this benefit is often associated with an inadvertent increase in toxic side effects and no subsequent change in therapeutic index (a representation of margin of safety).2 Consequently, retrometabolic drug design improves therapeutic index through rapid conversion of the active drug to inactive metabolites. Overall, this approach maximizes local efficacy, minimizes systemic toxicity, and enhances organ specificity.
Retrometabolic drug design can be divided into two classes: soft drugs and chemical delivery systems (CDS) (Figure 1).2 CDS involves biologically inert molecules that require several step-by-step chemical reactions to convert to an active compound. Thus, they are enzymatically converted into an active compound to exert a local desired therapeutic effect,
 which is then followed by subsequent enzymatic deactivation. In contrast, soft drugs are active therapeutic agents that are derived from lead compounds, which are molecules with known chemical structures and activity.1 Soft drugs are characterized by having a metabolically sensitive moiety, which is a structural modification to the lead compound. This metabolically sensitive moiety is specifically designed to allow for rapid predictable metabolism into inactive metabolites. Consequently, soft drugs produce rapid metabolism as they rely on controlled hydrolytic deactivation by enzymes that are distributed ubiquitously throughout the body. Soft drugs can often be confused with prodrugs. The key difference between soft drugs and prodrugs is that soft drugs become inactivated through metabolic reactions, while prodrugs undergo metabolic reactions to become activated.1
Soft drugs, in particular, offer an encouraging therapeutic alternative for the field of dermatology given that topical drug application can still allow for systemic drug absorption. Since skin conditions targeted by dermatologic therapies are often not severe, drug design should consider maximizing the riskbenefit ratio and thereby minimizing adverse effects.3 An ideal soft drug for dermatologic use should be slowly metabolized in the skin, yet rapidly converted to inactive moieties in other sites. Thus, dermatologic soft drugs should exhibit the following characteristics as described by Aprile et al3:
• Low skin clearance for maximal therapeutic effect
• High systemic clearance to avoid systemic toxicity
• Metabolism mediated through hydrolysis or enzymes with differential expression depending on organ or tissue
• Generation of inactive metabolites
Brief Overview of Soft Drugs in Other Medical Specialties
Retrometabolic drug design has offered promising alternative treatment options in multiple medical specialties, particularly in ophthalmology. Clinically approved soft drugs include loteprednol etabonate (soft corticosteroid), esmolol and landiolol (soft beta-blocker), remifentanil (soft opioid analgesic), and clevidipine (soft calcium-channel blocker).4 Loteprednol etabonate and esmolol are discussed in this section.
Although topical corticosteroids are often the mainstay therapy for ophthalmic disorders, they can cause significant systemic toxicity through the formation of multiple steroid metabolites. Furthermore, topical corticosteroids can produce a number of ocular complications, such as elevation of intraocular pressure (IOP), steroid-induced glaucoma, and cataract formation among others.5 Thus, the development of the soft corticosteroid, loteprednol etabonate, posed an advantageous alternative to minimize corticosteroid-induced
which is then followed by subsequent enzymatic deactivation. In contrast, soft drugs are active therapeutic agents that are derived from lead compounds, which are molecules with known chemical structures and activity.1 Soft drugs are characterized by having a metabolically sensitive moiety, which is a structural modification to the lead compound. This metabolically sensitive moiety is specifically designed to allow for rapid predictable metabolism into inactive metabolites. Consequently, soft drugs produce rapid metabolism as they rely on controlled hydrolytic deactivation by enzymes that are distributed ubiquitously throughout the body. Soft drugs can often be confused with prodrugs. The key difference between soft drugs and prodrugs is that soft drugs become inactivated through metabolic reactions, while prodrugs undergo metabolic reactions to become activated.1
Soft drugs, in particular, offer an encouraging therapeutic alternative for the field of dermatology given that topical drug application can still allow for systemic drug absorption. Since skin conditions targeted by dermatologic therapies are often not severe, drug design should consider maximizing the riskbenefit ratio and thereby minimizing adverse effects.3 An ideal soft drug for dermatologic use should be slowly metabolized in the skin, yet rapidly converted to inactive moieties in other sites. Thus, dermatologic soft drugs should exhibit the following characteristics as described by Aprile et al3:
• Low skin clearance for maximal therapeutic effect
• High systemic clearance to avoid systemic toxicity
• Metabolism mediated through hydrolysis or enzymes with differential expression depending on organ or tissue
• Generation of inactive metabolites
Brief Overview of Soft Drugs in Other Medical Specialties
Retrometabolic drug design has offered promising alternative treatment options in multiple medical specialties, particularly in ophthalmology. Clinically approved soft drugs include loteprednol etabonate (soft corticosteroid), esmolol and landiolol (soft beta-blocker), remifentanil (soft opioid analgesic), and clevidipine (soft calcium-channel blocker).4 Loteprednol etabonate and esmolol are discussed in this section.
Although topical corticosteroids are often the mainstay therapy for ophthalmic disorders, they can cause significant systemic toxicity through the formation of multiple steroid metabolites. Furthermore, topical corticosteroids can produce a number of ocular complications, such as elevation of intraocular pressure (IOP), steroid-induced glaucoma, and cataract formation among others.5 Thus, the development of the soft corticosteroid, loteprednol etabonate, posed an advantageous alternative to minimize corticosteroid-induced
side effects. Loteprednol etabonate is derived from the lead compound, cortienic acid, which is a known inactive metabolite of hydrocortisone. This soft corticosteroid is created by the addition of 17α and 17β carbonates or ethers to cortienic acid.5 This structural design is particularly beneficial in comparison to topical corticosteroids given its lack of intraocular pressure elevation. Loteprednol etabonate received FDA approval in 1998 for use in all inflammatory and allergy-related ophthalmic disorders, including allergic conjunctivitis, uveitis, and postcataract surgery among others.5 A 28-day study assessing the efficacy of loteprednol etabonate showed that significantly fewer patients exhibited IOP elevation greater than 10 mmHg in comparison to those receiving prednisolone acetate therapy (1.7% vs 6.7%, respectively).5 Loteprednol etabonate is currently being investigated for the treatment of asthma, rhinitis, and colitis, given its anti-inflammatory effects.
Retrometabolic drug design has also played a critical role in cardiology with the development of soft beta blockers. Traditional beta-blockers can exhibit significant and multiple systemic effects. Thus, a short-acting beta-blocker for hypertensive emergencies is desired to prevent unwanted side effects. Thus, a short-acting soft beta-blocker, esmolol, was developed and received FDA approval in 1986 for intravenous clinical use.6 Furthermore, a highly potent and cardioselective beta-blocker, landiolol (ONO-1101), has been developed. Its structural modifications include a morpholinocarbonylamino moiety and has S-configured hydroxy, which allows for nine times more potent β-antagonist activity and eight times more potent cardioselectivity than esmolol.6
Use of Retrometabolic Drug Design in Dermatology
Soft phosphodiesterase-4 (PDE) inhibitors
PDE4 inhibitors target the PDE4 receptor, which has been associated with various inflammatory diseases. However, traditional PDE4 inhibitors are often accompanied by a host of adverse effects, especially in the gastrointestinal system.3 Topical application mitigates the burden of these gastrointestinal side effects. Crisaborole (AN2728) is a topical benzoxaborole that received FDA approval in 2016 for the treatment of atopic dermatitis in individuals older than 2 years of age.3 Clinical trials on crisaborole have shown that 2% ointment applied twice daily can alleviate symptom severity with drug activity in the epidermis and dermis followed by rapid hydrolysis upon entry into the bloodstream.3 Currently, other benzoxaborole derivatives with a metabolically sensitive ester moiety are being investigated. Phase II trials are currently underway for lotamilast, which contains a methyl ester that rapidly metabolizes the drug to carboxylic acid.3 Preclinical data for compound LEO-29102 have demonstrated that the drug is well-tolerated in animal models and displays fast pharmacokinetic activity with low systemic levels of active compound.3
Soft Janus kinase (JAK) inhibitors
Retrometabolically designed drugs have also shown promising results for treatment of psoriasis and other inflammatory conditions. The chronic inflammation from psoriasis can be related to interactions of intracellular signaling pathways, such as signaling by the Janus kinase (JAK) family of tyrosine kinases.7 JAK inhibitors have been previously investigated for the treatment of other chronic inflammatory conditions, such as rheumatoid arthritis and myelofibrosis. However, currently available JAK inhibitors, including topical options, can produce significant immunosuppression and cytopenias leading to life-threatening infections.3 In order to mitigate these side effects, soft JAK inhibitors are desired. A fragmentbased screen was performed in 2016, which isolated a fragment with an indazole hit from the lead compound. The soft drug was developed by adding a phenol moiety at the 6’-position and sulfonamide moieties.7 These soft agents exhibit similar potency to traditional topical JAK inhibitors, but still require further modification as they were found to be phototoxic and unstable in light.7
Soft capsaicinoids
New capsaicinoids are currently in the preclinical phases of development for the treatment of inflammatory and pruritic dermatologic conditions. Traditional capsaicins can cause multiple systemic toxicities due to their pharmacological activity at the transient receptor potential vanilloid (TRPV1), a calcium permeable non-selective ion channel, which plays a role in pain physiology and neurogenic inflammation.8 The most common side effect is hyperthermia, while chronic application can increase skin carcinogenesis. The lipophilic and non-water soluble nature of capsaicin allows the drug compound to remain in human skin for long periods of time without being metabolized, which can lead to erythema reactions.8 Consequently, capsaicin soft drugs that can be rapidly hydrolyzed by esterases were desired to minimize the significant side effects associated with traditional capsaicinoids. A soft drug of capsaicin was developed through the addition of an ester group to the lipophilic tail of the lead compound via the Passerini adduct, which contains an amide and an unstable ester moiety.8 This unstable ester moiety increases susceptibility to rapid in situ hydrolytic deactivation. The soft capsaicinoids have minimized the “burning” sensation and hyperthermia of the traditional drug. In addition, variations in molecule length may control whether the compound acts solely in the epidermis or penetrates deeper layers of the skin.8
Soft estrogens
Soft estrogens have been studied for the management of cutaneous aging. Decreased circulating estrogens have been hypothesized to contribute to skin atrophy, changes in pigmentation, and wrinkling.9 Overall, the benefits of
 estrogen on the skin include increased extracellular matrix components, increased moisture retention, and increased thickness of the dermis and epidermis.9 However, estrogen can cause systemic toxicity, such as increased risk for breast cancer, endometrial cancer, and stroke. Thus, soft estrogens offer a therapeutic option for cutaneous aging that avoids unwanted side effects due to their rapid metabolism by ubiquitously distributed plasma esterases in the hypodermis.9 In addition, soft estrogens undergo metabolism without the cytochrome P450 system, which reduces the possibility of drug-drug interactions. Patents currently exist for estradiol-16α-carboxylic acid esters and 15α-carboxylic acid esters that act as “soft estrogens.â€9 Although these soft estrogens are still in the development stages, the data from preliminary animal models show promising evidence for future use.
Soft sphingosine-1-phopshate receptor 1 (S1PR1) modulators
S1PR1 agonists are also being developed for the treatment of psoriasis. Traditional forms of S1PR1 agonists can cause severe adverse events ranging from lymphopenias* to bradycardia, which has limited their dermatologic use.3 A soft S1PR1 agonist, ponesimod, reached Phase II clinical trials for management of severe plaque psoriasis; however, the compound did not display significant activity at the S1PR1 site.3 Subsequently, a second more successful attempt identified a metabolically sensitive phenol moiety, which promoted rapid metabolism through hydrolysis in the skin and conjugation with glucuronic acid in the liver.3 The S1PR1 agonists are still in the preclinical stages of investigation.
Emerging Evidence for the Use of Sofpironium Bromide to Treat PAH
With an estimated prevalence of 4.8%, hyperhidrosis is a skin disease that involves excessive sweat production than is needed to maintain thermal homeostasis. This condition is due to excessive cholinergic stimulation of eccrine sweat glands with resultant acetylcholine activity at local postsynaptic muscarinic M3 receptors that induces sweating.10 Current treatment recommendations include glycopyrronium cloth (a topical anticholinergic therapy), botulinum toxin type A, and topical aluminum chloride for the treatment of PAH.10,11 However, these options are not ideal as glycopyrronium cloth can still cause systemic adverse effects, botulinum toxin requires repeated injections, and aluminum chloride has not been well-studied in randomized controlled trials.10,11 Consequently, a soft drug approach offers an excellent solution for the treatment of PAH.
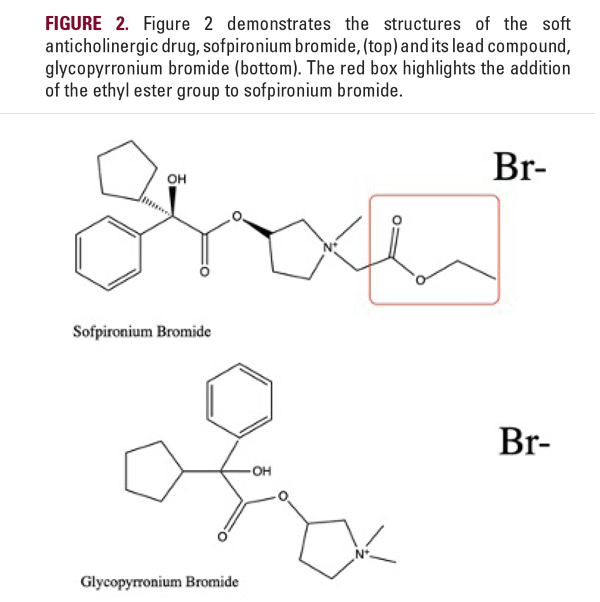
Soft anticholinergics are currently being studied for the treatment of PAH. The significant side effect profile of traditional anticholinergics, including topical options, often limits their use. To minimize systemic effects associated with anticholinergic drugs, a soft drug design for anticholinergics is highly desired. The soft anticholinergic sofpironium bromide is derived from a known muscarinic (M3) receptor antagonist, glycopyrrolate, which is a quaternary ammonium compound with poor blood-brain permeability and thus reduced central nervous system activity.12 Soft anticholinergic analogs are created by adding an ethyl ester analog to a known lead compound and shortening the two to three carbon atom bridge separating the quaternary head from the ester function of the “hard†anticholinergic drug to a one-carbon bridge (Figure 2).13,14 The ethyl ester analog allows for rapid non-enzymatic hydrolysis of the active compound to inactive metabolites upon entry into the systemic circulation.12 This structural change exemplifies the key principle of retrometabolic drug design as it promotes faster degradation of active molecules following the drug’s pharmacological activity at the target site. The formulation of soft anticholinergics is based on stabilization through direct transesterification.13 Since stereospecificity plays an important role at muscarinic receptors, two new N-substituted glycopyrrolate analogs that are stereoisomers of traditional glycopyrrolate were created.15 The new soft anticholinergic agents were also found to be more susceptible to extrahepatic metabolism, which prevents unwanted drug-drug interactions through the cytochrome P450 mechanism.12
Currently, most of the available data regarding the use of sofpironium bromide for the treatment of PAH is from Phase II and Phase III clinical trials in the United States and Japan. Sofpironium bromide is currently under investigation in the United States and approved in Japan for treatment of PAH.
estrogen on the skin include increased extracellular matrix components, increased moisture retention, and increased thickness of the dermis and epidermis.9 However, estrogen can cause systemic toxicity, such as increased risk for breast cancer, endometrial cancer, and stroke. Thus, soft estrogens offer a therapeutic option for cutaneous aging that avoids unwanted side effects due to their rapid metabolism by ubiquitously distributed plasma esterases in the hypodermis.9 In addition, soft estrogens undergo metabolism without the cytochrome P450 system, which reduces the possibility of drug-drug interactions. Patents currently exist for estradiol-16α-carboxylic acid esters and 15α-carboxylic acid esters that act as “soft estrogens.â€9 Although these soft estrogens are still in the development stages, the data from preliminary animal models show promising evidence for future use.
Soft sphingosine-1-phopshate receptor 1 (S1PR1) modulators
S1PR1 agonists are also being developed for the treatment of psoriasis. Traditional forms of S1PR1 agonists can cause severe adverse events ranging from lymphopenias* to bradycardia, which has limited their dermatologic use.3 A soft S1PR1 agonist, ponesimod, reached Phase II clinical trials for management of severe plaque psoriasis; however, the compound did not display significant activity at the S1PR1 site.3 Subsequently, a second more successful attempt identified a metabolically sensitive phenol moiety, which promoted rapid metabolism through hydrolysis in the skin and conjugation with glucuronic acid in the liver.3 The S1PR1 agonists are still in the preclinical stages of investigation.
Emerging Evidence for the Use of Sofpironium Bromide to Treat PAH
With an estimated prevalence of 4.8%, hyperhidrosis is a skin disease that involves excessive sweat production than is needed to maintain thermal homeostasis. This condition is due to excessive cholinergic stimulation of eccrine sweat glands with resultant acetylcholine activity at local postsynaptic muscarinic M3 receptors that induces sweating.10 Current treatment recommendations include glycopyrronium cloth (a topical anticholinergic therapy), botulinum toxin type A, and topical aluminum chloride for the treatment of PAH.10,11 However, these options are not ideal as glycopyrronium cloth can still cause systemic adverse effects, botulinum toxin requires repeated injections, and aluminum chloride has not been well-studied in randomized controlled trials.10,11 Consequently, a soft drug approach offers an excellent solution for the treatment of PAH.
Soft anticholinergics are currently being studied for the treatment of PAH. The significant side effect profile of traditional anticholinergics, including topical options, often limits their use. To minimize systemic effects associated with anticholinergic drugs, a soft drug design for anticholinergics is highly desired. The soft anticholinergic sofpironium bromide is derived from a known muscarinic (M3) receptor antagonist, glycopyrrolate, which is a quaternary ammonium compound with poor blood-brain permeability and thus reduced central nervous system activity.12 Soft anticholinergic analogs are created by adding an ethyl ester analog to a known lead compound and shortening the two to three carbon atom bridge separating the quaternary head from the ester function of the “hard†anticholinergic drug to a one-carbon bridge (Figure 2).13,14 The ethyl ester analog allows for rapid non-enzymatic hydrolysis of the active compound to inactive metabolites upon entry into the systemic circulation.12 This structural change exemplifies the key principle of retrometabolic drug design as it promotes faster degradation of active molecules following the drug’s pharmacological activity at the target site. The formulation of soft anticholinergics is based on stabilization through direct transesterification.13 Since stereospecificity plays an important role at muscarinic receptors, two new N-substituted glycopyrrolate analogs that are stereoisomers of traditional glycopyrrolate were created.15 The new soft anticholinergic agents were also found to be more susceptible to extrahepatic metabolism, which prevents unwanted drug-drug interactions through the cytochrome P450 mechanism.12
Currently, most of the available data regarding the use of sofpironium bromide for the treatment of PAH is from Phase II and Phase III clinical trials in the United States and Japan. Sofpironium bromide is currently under investigation in the United States and approved in Japan for treatment of PAH.
The following sections expand on these studies.
Phase II clinical trials: United States
The Phase II, randomized, controlled, double-blinded trial conducted in the United States randomized 227 patients to once daily topical application of vehicle or sofpironium bromide gel at 5%, 10%, or 15% drug concentrations over a 6-week treatment period.16 The primary endpoint that determined treatment efficacy was a 1-point or greater improvement and a change on continuous measure on the Hyperhidrosis Disease Severity Measure – Axillary (HDSM-Ax) scale, which is a validated 11-item patient reported measure of symptom severity and frequency from baseline to end of treatment (EOT). Secondary efficacy endpoints were defined as a greater than 50% reduction in gravimetric sweat production (GSP), changes on the Hyperhidrosis Disease Severity Score (HDSS), and a modified Dermatology Life Quality Index (DLQI) from baseline to EOT. At the end of the study period, 70%, 79%, 76%, and 54% of patients in the 5%, 10%, 15%, and vehicle groups, respectively, significantly satisfied the criteria for the primary endpoint efficacy (P<0.05).16 Furthermore, all drug concentrations of sofpironium bromide gel exhibited significant reduction in GSP, with the 5% and 15% sofpironium bromide group experiencing meaningful change in comparison to the vehicle (P=0.01 and 0.04, respectively).16 In addition, the authors of the study observed that the 5% drug concentration group maintained the lowest anticholinergic adverse events in comparison to the higher drug concentration groups.10 The side effects were deemed to be mostly mild-to-moderate in severity. The study concluded that therapeutic efficacy was observed across all three drug concentrations. Given the tolerability and safety of the drug demonstrated in Phase II studies, the investigation of sofpironium bromide has progressed to Phase III trials.
Phase III clinical trials: Japan
A Phase III multi-center, randomized, double-blinded trial as well as a long-term extension (LTE) study in Japan also investigated the efficacy and safety of sofpironium bromide in subjects with PAH. The Phase III trial randomized 281 patients over 12 years of age to sofpironium bromide 5% gel or placebo over a 6-week treatment period.10 The primary endpoint, efficacy, was defined as the proportion of patients who satisfied both criteria of a 1 or 2 HDSS score and a 50% or more reduction in GSP. 53.9% of patients in the sofpironium bromide group achieved the primary endpoint in comparison to only 36.4% of the placebo group. There was a statistically significant difference of 17.5% (P=0.003) in the primary efficacy endpoint between the treatment and placebo groups.10 The treatment group also experienced a higher number of adverse events than the placebo group (44% vs 30.7%, respectively).10 However, the adverse events were mostly mild-to-moderate in severity with the most common (>5%) being application site dermatitis and nasopharyngitis.10 Notably, the clinical trials in Japan have only evaluated the 5% drug concentration of sofpironium bromide gel without investigating higher concentrations of the drug.
Long-term extension study: Japan
The LTE study evaluated sofpironium bromide 5% gel at 52 weeks in 185 patients who completed the original Phase III trial. Those who continued on sofpironium bromide from the original study were known as the “extension” group (n=91), while those who switched from placebo to sofpironium bromide were called the “switching” group (n=94).17 The primary endpoint was similar to that of the original study. 57.4% of the switching group and 58.2% of the extension group achieved an HDSS score of 1 or 2 and a greater than 50% reduction in GSP.17 80.9% of the switching group and 83.5% of the extension group exhibited drug-related adverse events.17 However, most adverse events were mild in nature (75.5% of the switching group and 75.8% of the extension group).17 The most common adverse events were nasopharyngitis and application site dermatitis, similar to those in the original Phase III study. Thus, the results from the Japan studies demonstrate sofpironium bromide, with its retrometabolic drug design, is effective and safe for the treatment of PAH.
Notably, sofpironium bromide gel, 5% (ECCLOCK®) is marketed in Japan for the treatment of PAH.
CONCLUSIONS
In summary, retrometabolic drug design promotes therapeutic
efficacy at the target site while minimizing systemic
toxicity. Soft drugs have proven useful across multiple
medical specialties, such as in ophthalmology with the soft
corticosteroid, loteprednol etabonate, for the treatment of
inflammatory eye disorders. Furthermore, retrometabolic
drug design has shown promising results in dermatology with
the development of soft JAK inhibitors, soft PDE4 inhibitors,
and more for the treatment of inflammatory and autoimmune
dermatologic diseases. Sofpironium bromide is the latest
development of soft drugs in the field of dermatology. The
encouraging evidence presented here from Phase II and Phase
III clinical trials in the United States and Japan demonstrates
the efficacy and safety of sofpironium bromide in the treatment
of PAH, highlighting the expanding use of retrometabolic drug
design within the field of dermatology.
DISCLOSURE
Dr. April Armstrong has served as a research investigator and/ or scientific advisor to AbbVie, ASLAN, BI, BMS, EPI, Incyte, Leo, UCB, Janssen, Lilly, Novartis, Ortho Dermatologics, Sun, Dermavant, Dermira, Sanofi, Regeneron, Pfizer, and Modmed.
Dr. Leon Kircik has served either as a consultant, speaker,
and advisory board member or an investigator for Brickell, Dermira, and Eli Lilly.
Deepak Chadha and Rasika Reddy have no conflicts of interest to report.
ACKNOWLEDGMENTS
We acknowledge Dr. Nicholas Bodor for his thoughtful
suggestions and guidance in the preparation of this
manuscript.
REFERENCES
1. Bodor NB, Buchwald P. Retrometabolism-Based Drug Design and Targeting. In: Burger’s Medicinal Chemistry and Drug Discovery, DJ Abraham, Editor. 2003, John Wiley & Sons, Inc.
2. Bodor N, Buchwald P. Recent advances in retrometabolic drug design (RMDD) and development. Pharmazie. 2010;65(6):395-403.
3. Aprile S, Serafini M, Pirali T. Soft drugs for dermatological applications: recent trends. Drug Discov Today. 2019;24(12):2234- 2246.
4. Bodor N, Buchwald P. Soft drug design: general principles and recent applications. Med Res Rev. 2000;0(1):58-101.
5. Comstock TL, Sheppard JD. Loteprednol etabonate for inflammatory conditions of the anterior segment of the eye: twenty years of clinical experience with a retrometabolically designed corticosteroid. Expert Opin Pharmacother. 2018;19(4):337-353.
6. Buchwald P, Bodor N. Recent advances in the design and development of soft drugs. Pharmazie; 2014;69(6):403-13.
7. Ritzén A, M.D. Sørensen, K.N. Dack, et al. Fragment-based discovery of 6-arylindazole JAK inhibitors. ACS Med Chem Lett; 2016;7(6):641-6.
8. Serafini M, Griglio A, Aprile S, et al. Targeting transient receptor potential vanilloid 1 (TRPV1) channel softly: The discovery of passerini adducts as a topical treatment for inflammatory skin disorders. J Med Chem. 2018;61(10):4436-4455.
9. Svoboda RM, Del Rosso JQ, Zeichner JA, Draelos ZD. Revisiting the beneficial effects of estrogen on the skin: a comprehensive review of the literature and a look to the future. J Cutan Med. 2018;2(5).
10. Yokozeki H, et al. A phase 3, multicenter, randomized, doubleblind, vehicle-controlled, parallel-group study of 5% sofpironium bromide (BBI-4000) gel in Japanese patients with primary axillary hyperhidrosis. J Dermatol. 2021;48(3):279-288.
11. Pariser D. What’s new in the management of hyperhidrosis. In Fall Clinical Dermatology Conference. 2021. Las Vegas, NV.
12. Ji F, Wu W, Dai X, et al. Synthesis and pharmacological effects of new, N-substituted soft anticholinergics based on glycopyrrolate. J Pharm Pharmacol. 2005;57(11):1427-35.
13. Bodor N, Woods R, Raper C, et al. Soft drugs. 3. A new class of anticholinergic agents. J Med Chem. 1980;23(5):474-80.
14. Paik J. Sofpironium bromide: First approval. Drugs. 2020; 80(18):1981-1986.
15. Tóth-Sarudy E, Tóth G, I Pallagi I, et al. Preparation and biological effects of pure stereoisomeric novel soft anticholinergics. Pharmazie. 2006; 61(2):90-6.
16. Kirsch B, Smith S, Cohen J, et al. Efficacy and safety of topical sofpironium bromide gel for the treatment of axillary hyperhidrosis: A phase II, randomized, controlled, double-blinded trial. J Am Acad Dermatol. 2020;82(6):1321-1327.
17. Fujimoto T, Abe Y, Igarashi M, et al. A phase III, 52-week, open-label study to evaluate the safety and efficacy of 5% sofpironium bromide (BBI-4000) gel in Japanese patients with primary axillary hyperhidrosis. J Dermatol. 2021;48(8):1149-1161.
AUTHOR CORRESPONDENCE
April Armstrong MD MPH Armstrongpublication@gmail.com






