INTRODUCTION
Anti-cancer therapies (ACTs) cause physically and psychosocially distressing dermatologic side effects (DSEs) that occasionally necessitate dose reduction or treatment discontinuation.1 While their psychological impact is well-established, limited data exist on public perceptions and knowledge of DSEs.
To evaluate knowledge and perceptions of ACT DSEs in a medically underserved community, an IRB-approved survey (#NCR191384) was offered to all attendees (>18 years) at two health fairs in Southeast Washington, D.C., the most medically underserved area of the district. ACT was defined as any anticancer medication and/or radiation. Assessed DSEs included hair loss (HL), dry skin/rash, and nail changes (NCs). The survey was completed by 77 attendees (65% response). The majority were female (88.3%), 45-54 years old (23.7%), and Black or African American (71.5%). Twenty-one percent of respondents were previously treated for cancer.
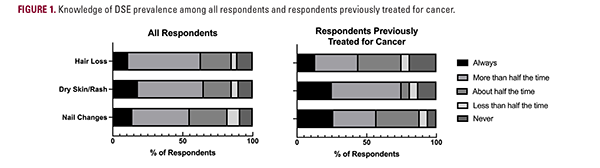
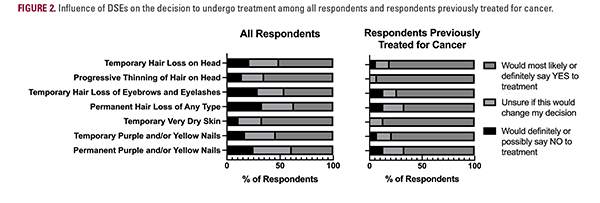
Most respondents believed that ACTs cause HL (52% [all respondents] vs 31% [respondents previously treated for cancer]), dry skin/rash (47% vs 50%), and NCs (41% vs 31%) more than half the time (Figure 1). The DSEs that respondents most frequently reported would make them possibly/definitely not undergo treatment were permanent HL (33% vs 13%), temporary eyebrow/eyelash HL (27% vs 13%), and permanent nail discoloration (24% vs 13%; Figure 2). Notably, half of the patients who were previously treated for cancer did not visit a dermatologist during cancer treatment.
To evaluate knowledge and perceptions of ACT DSEs in a medically underserved community, an IRB-approved survey (#NCR191384) was offered to all attendees (>18 years) at two health fairs in Southeast Washington, D.C., the most medically underserved area of the district. ACT was defined as any anticancer medication and/or radiation. Assessed DSEs included hair loss (HL), dry skin/rash, and nail changes (NCs). The survey was completed by 77 attendees (65% response). The majority were female (88.3%), 45-54 years old (23.7%), and Black or African American (71.5%). Twenty-one percent of respondents were previously treated for cancer.
Most respondents believed that ACTs cause HL (52% [all respondents] vs 31% [respondents previously treated for cancer]), dry skin/rash (47% vs 50%), and NCs (41% vs 31%) more than half the time (Figure 1). The DSEs that respondents most frequently reported would make them possibly/definitely not undergo treatment were permanent HL (33% vs 13%), temporary eyebrow/eyelash HL (27% vs 13%), and permanent nail discoloration (24% vs 13%; Figure 2). Notably, half of the patients who were previously treated for cancer did not visit a dermatologist during cancer treatment.