INTRODUCTION
Atopic Dermatitis (AD) is a chronic inflammatory skin disease that characteristically demonstrates upregulation of type 2 immune responses, impaired skin barrier function, and increased Staphylococcus aureus colonization.1,2 Intense and persistent pruritus is a defining feature of moderate-to-severe atopic dermatitis.3 AD has canonically been thought of as a disease that starts in childhood and rarely persists into adulthood.4 More recent work has shown that adult-onset AD is not an uncommon occurrence and that there is increasing evidence of lifelong, persistent disease that can extend beyond childhood.5,6 There appear to be important racial differences in AD prevalence, incidence, severity, and persistence in the United States (US).7 Indeed, African American (AA) and Latinx people in the US appear to experience higher rates of AD prevalence and disease burden.8 The pathophysiology of AD also appears to have differences racially; FLG mutations are 6 times less common in AA patients compared to European American patients with AD.7 Furthermore, while all ethnic groups of patients with AD have an immune phenotype characterized by strong TH2 activation, there appears to be significant difference in immune phenotype polarization, wherein Asian patients with AD have stronger TH17/TH22 activation and AA patients with AD have the highest serum IgE levels and largely lack TH1 and TH17 activation.7
Dupilumab is an IgG-4 based monoclonal antibody specific for IL-4Rα that is approved by the US Food and Drug Administration (FDA) for the treatment of refractory, moderate-to-severe AD in patients aged 6 months of age and older.9 Long-term real-world side effects of dupilumab, dupilumab-associated adverse events (d-AE), most commonly include ocular and cutaneous reactions.10 Given the recent findings of ethnic differences in immunophenotype characterization, it is of clinical interest whether d-AE differ by ethnicity. We aim to identify the most frequently reported d-AE and investigate the relative risk between the prevalence of d-AE and race in a single-center, retrospective chart review.
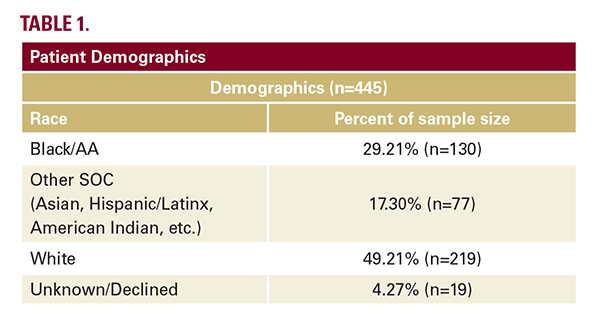
The inclusion criteria for this retrospective chart review were males and females aged 18 or older with a past or current prescription for dupilumab to treat dermatologic conditions, seen in the clinic between January 1, 2017, and January 1, 2022. Specifically, we analyzed the charts of atopic dermatitis patients 18 and older, who were seen at a University of Pennsylvania Health System (UPHS). Our goal was to identify the most common side effects in our cohort of Black/AA patients treated with dupilumab. Patients were excluded if they did not meet the inclusion criteria or if they were on dupilumab for an investigational trial or an off-label indication. This study was approved by the University of Pennsylvania Institutional Review Board. Patient demographics can be seen in Table 1.
Dupilumab is an IgG-4 based monoclonal antibody specific for IL-4Rα that is approved by the US Food and Drug Administration (FDA) for the treatment of refractory, moderate-to-severe AD in patients aged 6 months of age and older.9 Long-term real-world side effects of dupilumab, dupilumab-associated adverse events (d-AE), most commonly include ocular and cutaneous reactions.10 Given the recent findings of ethnic differences in immunophenotype characterization, it is of clinical interest whether d-AE differ by ethnicity. We aim to identify the most frequently reported d-AE and investigate the relative risk between the prevalence of d-AE and race in a single-center, retrospective chart review.
The inclusion criteria for this retrospective chart review were males and females aged 18 or older with a past or current prescription for dupilumab to treat dermatologic conditions, seen in the clinic between January 1, 2017, and January 1, 2022. Specifically, we analyzed the charts of atopic dermatitis patients 18 and older, who were seen at a University of Pennsylvania Health System (UPHS). Our goal was to identify the most common side effects in our cohort of Black/AA patients treated with dupilumab. Patients were excluded if they did not meet the inclusion criteria or if they were on dupilumab for an investigational trial or an off-label indication. This study was approved by the University of Pennsylvania Institutional Review Board. Patient demographics can be seen in Table 1.
Compared with clinical trials, our study population had a greater representation of Black/AA patients with dupilumab-treated AD, but a similar safety profile-with ocular manifestations as the most frequently reported d-AE (Table 2).11,12 Most patients with AD did not report d-AE (79%, n=352). Of the 219 White patients, nearly 1 in 2 reported d-AE, whereas out of 130 Black/AA patients, only 1 in 3 reported d-AE. Our data found that Black/AA patients experienced a lower risk of having a d-AE (RR 0.78; 95% CI 0.46, 1.34; Table 3), however, it was not a statistically significant difference. The lack of statistical significance may be due to our small sample size.






