INTRODUCTION
Basal and squamous cell carcinomas are the most common cancers in the United States with an alarming 80% increase in recent years.1,2 This is likely to increase as the population ages.3 Mohs micrographic surgery (MMS), known for its low recurrence rates, is among the preferred techniques for resecting these skin tumors when complex, large, or recurrent.4 The subsequent cutaneous reconstruction plays a pivotal role in restoring form, function, and quality of life for patients post-MMS.5 The impact of altered appearance or reduced function or well-being underscores the importance of effective interventions.6 In anatomical sites with elevated cosmetic and functional risk (such as the face, scalp, hands, and feet), innovative approaches are essential.7,8 For elderly patients who are not suitable surgical candidates for autologous flaps, skin grafts, or second-intent healing is contraindicated, nonoperative techniques such as placental allografts, have emerged as a promising solution9 that improve cosmesis and preserve functional ability.10 Allografts promote dermal reconstruction and have demonstrated positive outcomes in various scenarios, including randomized controlled trials for hard-to-heal wounds,11-15 pediatric burns,16 and post-MMS defects.17 However, concerns regarding overall costs have hindered their widespread adoption.18,19
A cost-effectiveness analysis (CEA) was conducted to compare placental allografts with autologous tissue-based methods for defect reconstruction following MMS.20 This study aimed to assess both the monetary and non-monetary variables that affect the value of placental allografts as a treatment option in dermatologic surgery.
MATERIALS AND METHODS
Study Design
This retrospective comparative cohort study evaluated the outcomes of two methods of primary Mohs defect reconstruction, placental allograft vs autologous tissue-based repair. Specifically, it compared the average cost and effectiveness between dehydrated human amnion/chorion membrane (dHACM) (EpiFix; MIMEDX Group Inc., Marietta, GA) to skin flaps and autologous skin grafts. This analysis focused on defects with Mohs Appropriate Use Score greater than or equal to 7 that could not be closed primarily or second-intention healing not appropriate. Lower-risk defects were excluded, as were cases receiving primary closure, delayed closure, or no repair. Administrative practice databases were queried for patients undergoing Mohs surgery with same-day reconstruction between 2014 to 2018.17 Deidentified cost and outcome data related to the index reconstruction were gathered from electronic medical records (ModMed Dermatology, Modernizing Medicine, Boca Raton, FL) linked to automated charge captures. This study assumed the analytic perspective of the healthcare provider to calculate cumulative cost according to allowable fee schedules and excluded subject costs such as medications and co-pays. Ethical approval and waiver of informed consent for protocol AFSUR008.2; Pro00031033 was obtained from Advarra IRB, Columbia, MD.
Outcome Measures
A cost analysis of patient care postoperative day 0 through discharge was performed for all subjects. The primary endpoint was the cost difference between dHACM and Standard of Care (SOC) for initial reconstruction, post-repair sequelae, and cumulative cost of care. Cost profiles were verified using Current Procedural Terminology (CPT) codes from provider documentation. Patient encounters were audited for clinical event charges prior to determining the final cost estimates. Ancillary procedures (eg, hematoma evacuation, wound debridement, scar excision, laser, dermabrasion, corticosteroid injection, and additional reconstruction) were included in cost calculations. All monetary values were reported in US dollars ($), inflation-adjusted to 2024 using the US Consumer Price Index (CPI).21 Intervention effectiveness was defined as the proportion of patients without any adverse post-repair sequelae in this study. These events included bleeding, wound infection, wound dehiscence, hematoma, flap or graft necrosis, graft loss, outside referral, donor site infection, persistent wound or scar erythema, distortion of surrounding tissue, and widened, hypertrophic, or keloid scarring. A subgroup analysis focused on specific complications; the total incidence of wound infection, hematoma, and flap or graft necrosis. Clinical documentation and ICD-10-CM coding (International Classification of Diseases, Tenth Revision, Clinical Modification) were used to investigate these metrics. The incremental cost-effectiveness ratio (ICER) was calculated as a measure of intervention effectiveness value, ICER = (Average cost A - Average cost B) / (Effectiveness A - Effectiveness B), where A and B represent dHACM vs SOC interventions, respectively.
Propensity Score Methods
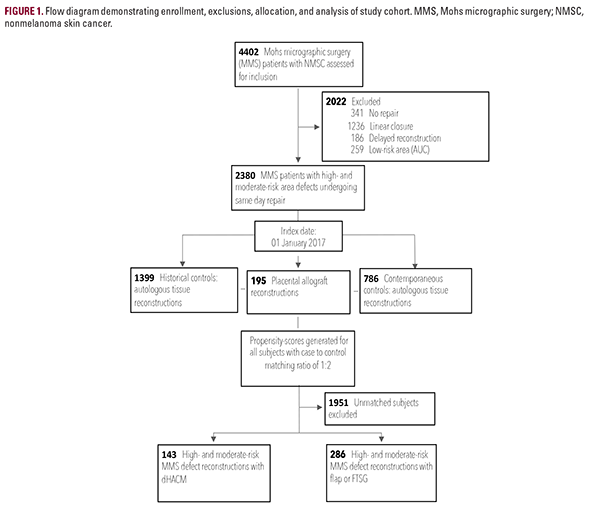
A propensity-score-matching model was constructed to ensure covariate balance before making statistical comparisons. The dependent variable was the method of reconstruction, placental allograft vs autologous tissue. Potential confounders (covariates) included patient demographics (age, sex, and functional status), comorbidities, Charlson Comorbidity Index (CCI) score, smoking status, tumor characteristics, number of extirpative stages performed, surgery time (minutes), and defect size (cm2). To mitigate confounding by time biases, the placental allograft initiation date was used to delineate historical and contemporary controls (before vs on or after 01 January 2017) from within the 5-year control arm continuum. Propensity scores were estimated using binary logistic regression and a greedy nearest-neighbor-matching algorithm without replacements. This strategy matched a placental allograft subject to an autologous tissue control (both historical and contemporaneous) with the nearest propensity score, yielding a case-control ratio of 1:2 without sacrificing precision.
Statistical Analysis
Continuous variable comparisons were evaluated using independent samples Student's t-test or Mann-Whitney U test, depending on distribution of data. Pearson's X2 test or Fisher's Exact test were used to compare categorical variables between groups and to assess differences in proportions. Multivariable linear regression was used to predict cost. Statistical significance was set at a two-tailed P value of < .05. All analyses were performed using SAS/STAT v9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Baseline Characteristics
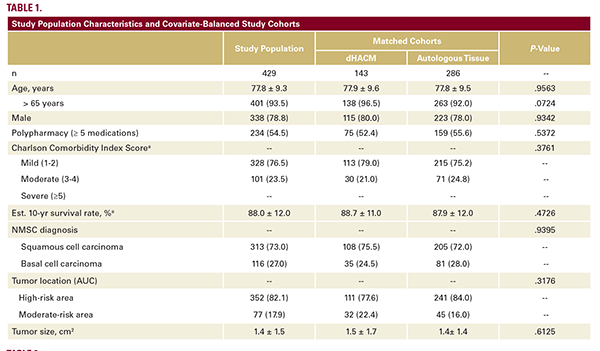
Of 4,402 Mohs surgery patients, 2,380 who underwent same-day reconstruction for high- and moderate-risk area defects met inclusion criteria. The final cohort consisted of 143 cases using dHACM and 286 autologous tissue controls (Figure 1). The covariate-balanced distributions are provided (Table 1). Among 429 patients, 335 were male (78.1%) and 401 were 65 years of age or older (93.5%). The mean patient age was 77.8 +/- 9.3
years. Over half (234/429; 54.5%) of the study population were routinely taking greater than or equal to 5 or more medications. The CCI severity of comorbidity burden in two-thirds (328/429; 76.5%) of individuals was mild. The CCI estimated mean 10-year survival rate was 88.0% +/- 12.0%. Tumor size (cm2), area of reconstructive risk, and the distributions of squamous cell vs basal cell carcinoma tumors were similar between groups (P>0.05).
Direct Cost Comparisons
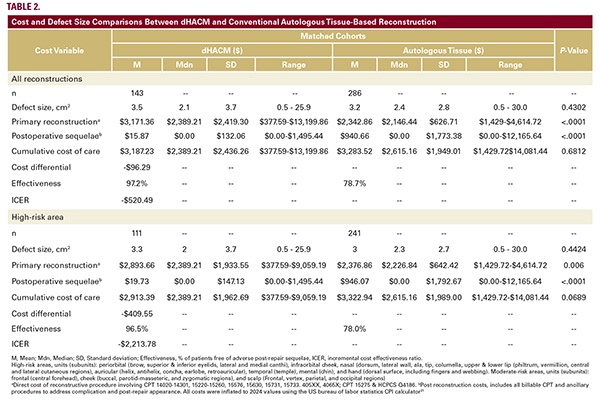
For primary reconstruction (POD, postoperative day 0), allotransplantation of dHACM vs SOC was more costly in all areas combined ($3171.36 vs $2342.86, P<0.0001), and highrisk areas ($2,893.66 vs $2376.86; P=0.0060; Table 2). When comparing the cost of post-repair sequelae (POD 1 through discharge), dHACM patients incurred significantly lower costs than autologous tissue patients in all areas combined ($15.87 vs $940.66, P<.0001), and in high-risk areas ($19.73 vs $946.07, P<.0001). No significant differences were found in cumulative cost comparisons between dHACM cases ($3,187.23) and control counterparts ($3,283.52) (P=0.6812).
Intervention Effectiveness
Overall, dHACM patients experienced significantly less adverse post-repair sequelae, 2.8% vs 21.3% (P<.0001; Figure 2). Specifically, dHACM was associated with a significantly lower incidence of infection (P=.0114), dehiscence (P=0.4189), flap or graft necrosis (P=0.0349), and hematoma (P=0.0066). Method of reconstruction did not impact outpatient visit frequency (P=0.2185). Distinctively, dHACM patients underwent fewer scar excisions than SOC (flap and graft) counterparts (P=0.0044). This resulted in a cumulative cost for dHACM treatment that was lower for high-risk patients where average cumulative
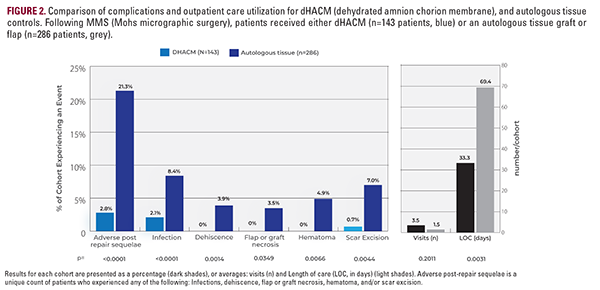
costs were reduced by $409.55. Additionally, reconstructions managed with dHACM reached their final disposition at a much faster rate. The average length of care (LOC) for dHACM differed significantly when compared to autologous tissue controls, 33.3 +/- 35.2 days vs 48.3 +/- 69.4 days, respectively (P=0.0031). Only 1.4% (2/143) of the dHACM group required care > 90 days vs 10.8% (31/286) of controls (P=0.0005; Figure 2).
Predictors of Greater Cost
A significant multiple regression model with all predictors was produced R2 = .398, F (19,409) = 15.86, P<0.001. Regression analysis revealed a cost increase of $1,449.60 (P<0.0001) for every instance of infection, and an additional $2,196.60 (P<0.0001) for each instance of flap or graft necrosis. A previous Mohs reconstruction at the study index site was also predictive within the model, increasing care totals by $625.30 (P=0.0174). Lastly, defect size was associated with greater cost by adding $98.80 for every additional cm2 of wound treated > 1.0 cm2.
Cost-Effectiveness
The average cost of primary reconstruction for each method was calculated.17 When compared to SOC, the intervention effectiveness of dHACM was significantly better, 97.2% vs 78.7% (P<0.0001; Figure 2, Table 2). The ICER calculation for dHACM was: -$96.29 / 18.5% incremental improvement in intervention effectiveness. dHACM is thus a dominant treatment, $520.49 is saved per complication-free individual (Table 2).
Example Cosmesis Results
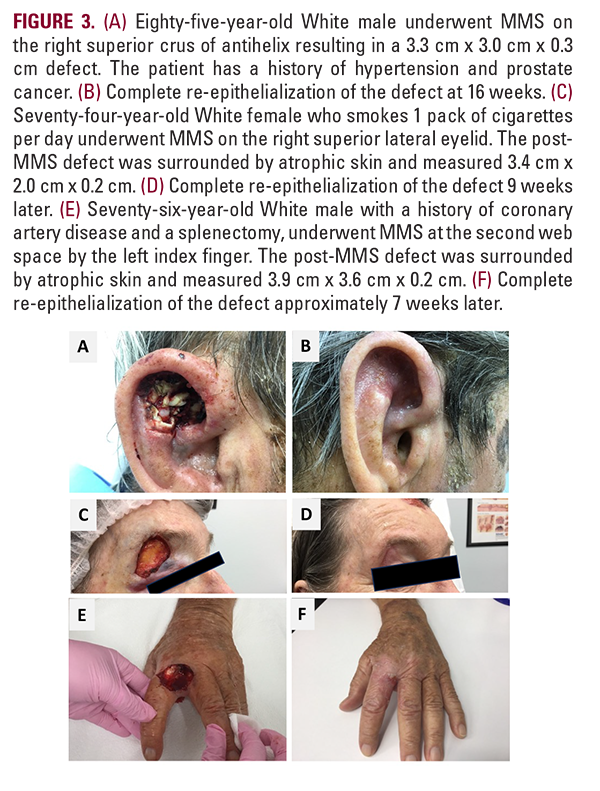
High-risk areas such as the superior crus of antihelix in an 85-year-old white male, the right superior lateral eyelid (eyebrow) in a 74-year-old White female, or the 2nd web space by the left index finger in a 76-year-old White male are shown (Figure 3). DHACM was applied every one to two weeks from the date of the Mohs surgery to re-epithelialization. Closure occurred in 7 to 16 weeks for the presented cases without complications.
DISCUSSION
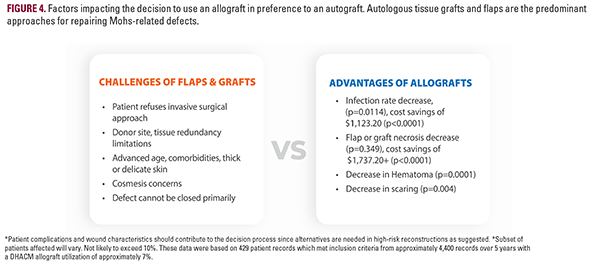
Our primary finding is that purchasing a placental allograft accounts for an average of 76.4% ($2,442.70) of the total reconstruction cost or $692.20 per cm2 of defect treated ($2,422.70 / 3.5 average defect cm2). Subjects treated with dHACM, avoided significant costly postoperative complications and ancillary procedures, while SOC patients incurred greater costs secondary to increased resource utilization (direct treatment of adverse sequelae, outpatient visits, aesthetic services) which offset the per-patient cumulative cost averages ($3,171.36 vs $2,342.86, P<0.0001). This trend was observed for the high-risk group and overall group (Table 2). A significantly longer length of care was identified in the autologous tissue group compared to allografts (48.3 +/- 69.4 days vs 33.3 +/- 35.2 days, P=0.0031), suggesting that additional time was required to successfully discharge these patients. dHACM patients also underwent fewer scar revisions (0.7% vs 7.0%, P=0.0044) or other adjunct cosmetic procedures commonly used to address appearance. Regression modeling identified autologous flap/ graft necrosis and infection as the greatest impacts on total cost. A significant predictive relationship between cost and a history of previous Mohs with reconstruction at the study index site suggests donor site tissue quality may impact the success of autologous tissue-based reconstructions. Best use cases for dHACM include: defects greater than 3.0 cm in a moderate to high-risk functionally and cosmetically sensitive area such as the superior crus of antihelix (3A, 3B), particularly when imbibition is inadequate for a full-thickness skin graft to survive, and when the wound exhibits exposed cartilage which must be protected as it is an avascular entity. Patients who smoke (3C, D) also have an increased risk of flap or graft failure due to hypoxia with the potential for an ensuing cascade of necrosis, inflammation, infection risk, pain, scar tissue formation, impaired cosmesis, and function impairment which can affect both the Primary and Secondary donor site defects, prolonging wound care. Although not analyzed in this study, dHACM may be beneficial when 1) side-to-side repair is not feasible, 2) there is limited tissue redundancy adjacent to the wound (low flap feasibility), 3) where secondary intention is a sub-optimal option, 4) or when patients with significant comorbidities demonstrate other risks for post-MMS complications (Figure 4). An allograft may be warranted at highly dynamic locations that are heavily utilized and traumatized such as the hands and digits (Figure 3E, 3F), where development of vessels to supply an autograft is challenged. The additional potential for adhesions at the exposed tendon at the base of this wound may warrant an allograft. Lastly, healing via secondary intention would create difficulty in daily dressing changes and wound cleaning for this patient to perform one-handed if a caregiver is not available. While autologous skin reconstruction remains the gold standard of treatment, as the patient population ages, we believe there will be a growing need for non-surgical reconstructive options.21 An elderly population will mean greater frailty and risk of recurrent resections and reconstructions limiting donor sites. Expectations of a good aesthetic outcome, timely closure, and a straightforward recovery remain important to an aging individual (Figure 3A-F).22,23 Economic literature on regenerative biomaterials is primarily in chronic wound management, traumatic wound reconstruction, or burn injury.23 The cost-benefit evidence is especially favorable for Medicare-aged patients with diabetic foot ulcers.24,25
Management with these advanced therapies is often associated with lowering overall medical costs through reduced utilization of resources and healthcare services.24,26 In the current study dHACM accounts for an average of 76.4% of total reconstruction costs; however, 98.3% (($15.87/$940.66) - 1) of downstream costs (complications) were averted for an overall reduction in cumulative costs. This cost savings is greatest in high-risk patients who would have more post-repair sequelae with an autologous graft (-$409.55, ICER is dominant). Patients of high or moderate-risk areas would save an average of $96.29 (ICER is dominant). Our data suggests that in elderly patients with wounds that cannot be closed primarily, dHACM appears to be a useful alternative to standard skin grafting and skin flaps with minimal financial tradeoff.
Study Limitations
This is not a randomized, controlled prospective study and thus causative correlations should not be implied but rather form the basis of testable hypotheses. Male gender was 78%. US research has shown that the incidence of basal cell carcinoma, the most frequent NMSC, has an incidence of 469 more cases per 100,000 person-years for men than women (a 1.46-fold increase).27 However, women were reported to be more sensitive to issues of cosmesis (odds ratio 2.84, P=0.009).17 Future studies should evaluate how gender impacts patient satisfaction. Our study based the effectiveness on the incidence of at least one adverse post-repair event when in reality, patients can experience more than one complication or outcome.
DISCLOSURES
DM and RAF are clinical researchers for MIMEDX Group, Inc. BH is a consultant to MIMEDX Group, Inc. The other authors, their immediate families, or any foundation with which they are affiliated have no conflicts of interest relevant to the subject matter of this manuscript.
Funding: Third-party data analysis was supported in part by the company, MIMEDX Group, Inc. through a clinical research agreement.
ACKNOWLEDGMENT
We would like to thank Georgina M. Michael for her thoughtful
data review and medical writing.
REFERENCES
- Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85(2):388-395. doi:10.1016/j.jaad.2021.03.109
- Albert A, Knoll MA, Conti JA, et al. Non-melanoma skin cancers in the older patient. Curr Oncol Rep. 2019;21(9). doi:10.1007/s11912-019-0828-9
- Zhang W, Zeng W, Jiang A, et al. Global, regional and national incidence, mortality and disability-adjusted life-years of skin cancers and trend analysis from 1990 to 2019: An analysis of the Global Burden of Disease Study 2019. Cancer Med. 2021;10(14):4905-4922. doi:10.1002/cam4.4046
- Garcovich S, Colloca G, Sollena P, et al. Skin cancer epidemics in the elderly as an emerging issue in geriatric oncology. Aging Dis. 2017;8(5):643-661. doi:10.14336/AD.2017.0503
- Chen A, Albertini JG, Bordeaux JS, et al. Evidence-based clinical practice guideline: reconstruction after skin cancer resection. Dermatol Surg. 2021;47(7):891-907. doi:10.1097/DSS.0000000000003115
- Sobanko JF, Sarwer DB, Zvargulis Z, et al. Importance of physical appearance in patients with skin cancer. Dermatol Surg. 2015;41(2):183-188. doi:10.1097/ DSS.0000000000000253
- Dey JK, Ishii LE, Joseph AW, et al. The cost of facial deformity: A health utility and valuation study. JAMA Facial Plast Surg. 2016;18(4):241-249. doi:10.1001/ jamafacial.2015.2365
- Egeler SA, Johnson AR, Ibrahim AMS, et al. Reconstruction of Mohs defects located in the head and neck. J Craniofacial Surg. 2019;30(2):412-417. doi:10.1097/SCS.0000000000005137
- Niknejad H, Peirovi H, Jorjani M, et al. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88-99. doi:10.22203/ecm.v015a07
- Mori S, Lee EH. Beyond the physician’s perspective: A review of patient-reported outcomes in dermatologic surgery and cosmetic dermatology. Int J Womens Dermatol. 2019;5(1):21-26. doi:10.1016/j.ijwd.2018.08.001
- Zelen CM, Gould L, Serena TE, et al. A prospective, randomised, controlled, multi-centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int Wound J. 2015;12(6):724-732. doi:10.1111/IWJ.12395
- Zelen CM, Serena TE, Gould L, et al. Treatment of chronic diabetic lower extremity ulcers with advanced therapies: a prospective, randomised, controlled, multi-centre comparative study examining clinical efficacy and cost. Int Wound J. 2016;13(2):272-282. doi:10.1111/IWJ.12566
- Zelen CM, Serena TE, Denoziere G, et al. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502-507. doi:10.1111/iwj.12097
- Bianchi C, Cazzell S, Vayser D, et al. A multicentre randomised controlled trial evaluating the efficacy of dehydrated human amnion/chorion membrane (EpiFix®) allograft for the treatment of venous leg ulcers. Int Wound J. 2018;15(1):114. doi:10.1111/IWJ.12843
- Serena TE, Carter MJ, Le LT, et al. A multicenter, randomized, controlled clinical trial evaluating the use of dehydrated human amnion/chorion membrane allografts and multilayer compression therapy vs multilayer compression therapy alone in the treatment of venous leg ulcers. Wound Repair and Regeneration. 2014;22(6):688-693. doi:10.1111/WRR.12227
- Ahuja N, Jin R, Powers C, et al. Dehydrated human amnion chorion membrane as treatment for pediatric burns. Adv Wound Care (New Rochelle). 2020;9(11):602-611. doi:10.1089/WOUND.2019.0983
- Toman J, Michael GM, Wisco OJ, et al. Mohs defect repair with dehydrated human amnion/chorion membrane. Facial Plast Surg Aesthet Med. 2022;24(1):48-53. doi:10.1089/FPSAM.2021.0167
- Morozzo U, Villafane JH, Ieropoli G, et al. Soft tissue reconstructions with dermal substitutes versus alternative approaches in patients with traumatic complex wounds. Indian J Surg. 2015;77(Suppl 3):1180-1186. doi:10.1007/ s12262-015-1235-6
- Chocarro-Wrona C, Lopez-Ruiz E, Peran M, et al. Therapeutic strategies for skin regeneration based on biomedical substitutes. JEADV. 2019;33(3):484-496. doi:10.1111/jdv.15391
- Burningham KM, Le K, He A, et al. Cost effectiveness of melanoma in situ resection and repair by dermatology compared to non-dermatology specialties at a single institution. Arch Dermatol Res. 2023;315(3):661-663. doi:10.1007/ s00403-022-02405-4
- US Bureau of Labor Statistics. CPI Inflation Calculator. US Bureau of Labor Statistics. Accessed February 25, 2024. https://www.bls.gov/data/inflation_ calculator.htm
- Cerrati EW, Thomas JR. Scar revision and recontouring post-Mohs surgery. Facial Plast Surg Clin North Am. 2017;25(3):463-471. doi:10.1016/j. fsc.2017.03.014
- Langer A, Rogowski W. Systematic review of economic evaluations of human cell-derived wound care products for the treatment of venous leg and diabetic foot ulcers. BMC Health Serv Res. 2009;9. doi:10.1186/1472-6963-9-115
- Tettelbach WH, Armstrong DG, Chang TJ, et al. Cost-effectiveness of dehydrated human amnion/chorion membrane allografts in lower extremity diabetic ulcer treatment. J Wound Care. 2022;31(Sup2):S10-S31. doi:10.12968/ JOWC.2022.31.SUP2.S10
- Armstrong DG, Tettelbach WH, Chang TJ, et al. Observed impact of skin substitutes in lower extremity diabetic ulcers: lessons from the medicare database (2015-2018). J Wound Care. 2021;30:S5-S16.
- Rice JB, Desai U, Ristovska L, et al. Economic outcomes among Medicare patients receiving bioengineered cellular technologies for treatment of diabetic foot ulcers. J Med Econ. 2015;18(8):586-595. doi:10.3111/13696998.2015.103 1793
- Wu S, Han J, Li WQ, Li T, Qureshi AA. Basal-cell carcinoma incidence and associated risk factors in US women and men. Am J Epidemiol. 2013;178(6):890. doi:10.1093/AJE/KWT073
AUTHOR CORRESPONDENCE
Gary S. Rogers MD Gary.Rogers@Lahey.org






