eMethods
Registry Design
The CorEvitas Psoriasis Registry questionnaires collect data on patient demographics, disease duration, medical history (including all prior and current treatments for psoriasis, smoking status, alcohol use, disease activity and severity, pain, as well as other clinician- and patient-reported outcomes, comorbidities and adverse events, infections, hospitalizations, and other targeted safety outcomes. Blood collection and other diagnostic tests are not required for participation; however, relevant standard of care laboratory and imaging results are reported when available. As of July 31, 2022, the registry includes 263 private and academic clinical sites with 591 physicians throughout 40 states in the US and 7 provinces in Canada. It collects data from both physicians (dermatologists) and patients at the time of outpatient clinical dermatological encounters. To date, CorEvitas has enrolled 17,804 patients with psoriasis representing 70,082 registry study visits.
Registry Inclusion Criteria
The CorEvitas Psoriasis Registry questionnaires collect data on patient demographics, disease duration, medical history (including all prior and current treatments for psoriasis, smoking status, alcohol use, disease activity and severity, pain, as well as other clinician- and patient-reported outcomes, comorbidities and adverse events, infections, hospitalizations, and other targeted safety outcomes. Blood collection and other diagnostic tests are not required for participation; however, relevant standard of care laboratory and imaging results are reported when available. As of July 31, 2022, the registry includes 263 private and academic clinical sites with 591 physicians throughout 40 states in the US and 7 provinces in Canada. It collects data from both physicians (dermatologists) and patients at the time of outpatient clinical dermatological encounters. To date, CorEvitas has enrolled 17,804 patients with psoriasis representing 70,082 registry study visits.
Registry Inclusion Criteria
- To be eligible for inclusion in CorEvitas Psoriasis Registry, the patient must:
- Have been diagnosed with psoriasis by a dermatologist.
- Be at least 18 years of age at the time of diagnosis.
- Be willing and able to provide written informed consent for participation in70, the registry.
- Have started on or switched to an eligible systemic psoriasis treatment at enrollment or within the previous 12 months of the date of enrollment.
- The interruption in treatment lasted <180 days; AND
- No other systemic psoriasis medications were initiated during the time period when the eligible medication was not in use.
If a subject has been prescribed but not started an eligible medication at the enrollment visit one of the following conditions must be met at the time of the first registry follow-up visit or within six months (180 days) of the enrollment visit, whichever occurs first:
Participating dermatologists are reimbursed for the collection of data at the time of routine clinical encounters. Enrollment data collection takes place on the day of the enrollment visit. Follow-up data collection takes place during CorEvitas follow-up visits which occur at the time of routine clinical encounters approximately every six months. Baseline period is the CorEvitas visit coinciding with therapy initiation within 42 days prior to, and inclusive of, the date of initiation. Data collected at follow-up visits cover the time period since the last CorEvitas visit, even if a routine or standard of care clinical encounter occurs between two CorEvitas visits. Routine clinical encounters conducted earlier than 150 days since the last CorEvitas visit are not eligible for reimbursement as a CorEvitas visit (though data collection may be completed and included in the registry dataset) unless the patient was prescribed or started a new eligible medication that the patient had not previously started within the previous 12 months.
Study Population
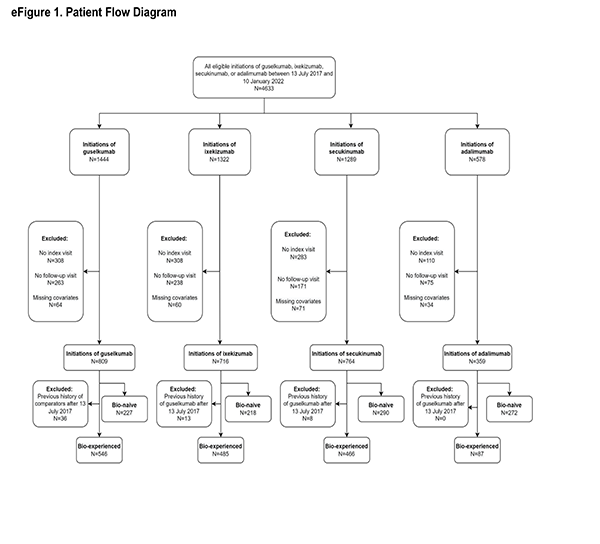
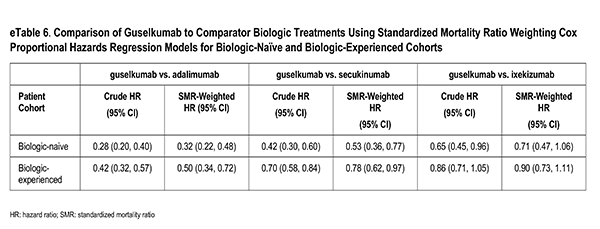
Patients initiating biologics of interest had to have a valid index date, defined as the CorEvitas visit within 42 days prior to and inclusive of the initiation date (i.e., index visit). Baseline characteristics were determined at index visit. Additionally, patients with no history of plaque psoriasis or patients with missing data in at least one of the propensity score (PS) model covariates were excluded from the analysis. Finally, patients initiating at a site in Canada were excluded from the analysis. Treatment and comparator groups were divided into biologic-naïve (i.e., no prior use of biologic therapies) and biologic-experienced (i.e., at least one prior biologic therapy) cohorts. Specific to the biologic-experienced cohorts, after July 13, 2017, biologic-experienced guselkumab initiators were excluded if prior to initiation of guselkumab they previously initiated any of the comparator drugs as a biologic-experienced initiation. This exclusion criterion ensured that a patient that had initiated any comparator drug prior to initiating guselkumab did not contribute to both the treatment and comparator cohorts. Furthermore, by excluding prior initiations of any of the comparator therapies, there was a single guselkumab cohort. Similarly, after July 13, 2017, specific to each biologic-experienced pairwise comparison: adalimumab, ixekizumab, or secukinumab initiators were excluded if prior to initiation of each respective comparator drug they initiated guselkumab as a biologic-experienced initiation. This exclusion criterion ensured that a patient that had initiated guselkumab prior to initiating the comparator did not contribute to both the treatment and comparator cohorts, thereby violating the independence of observations across treatments assumption.
- The eligible medication was started; OR
- Another eligible medication was started that has not been used in the previous 12 months.
Participating dermatologists are reimbursed for the collection of data at the time of routine clinical encounters. Enrollment data collection takes place on the day of the enrollment visit. Follow-up data collection takes place during CorEvitas follow-up visits which occur at the time of routine clinical encounters approximately every six months. Baseline period is the CorEvitas visit coinciding with therapy initiation within 42 days prior to, and inclusive of, the date of initiation. Data collected at follow-up visits cover the time period since the last CorEvitas visit, even if a routine or standard of care clinical encounter occurs between two CorEvitas visits. Routine clinical encounters conducted earlier than 150 days since the last CorEvitas visit are not eligible for reimbursement as a CorEvitas visit (though data collection may be completed and included in the registry dataset) unless the patient was prescribed or started a new eligible medication that the patient had not previously started within the previous 12 months.
Study Population
Patients initiating biologics of interest had to have a valid index date, defined as the CorEvitas visit within 42 days prior to and inclusive of the initiation date (i.e., index visit). Baseline characteristics were determined at index visit. Additionally, patients with no history of plaque psoriasis or patients with missing data in at least one of the propensity score (PS) model covariates were excluded from the analysis. Finally, patients initiating at a site in Canada were excluded from the analysis. Treatment and comparator groups were divided into biologic-naïve (i.e., no prior use of biologic therapies) and biologic-experienced (i.e., at least one prior biologic therapy) cohorts. Specific to the biologic-experienced cohorts, after July 13, 2017, biologic-experienced guselkumab initiators were excluded if prior to initiation of guselkumab they previously initiated any of the comparator drugs as a biologic-experienced initiation. This exclusion criterion ensured that a patient that had initiated any comparator drug prior to initiating guselkumab did not contribute to both the treatment and comparator cohorts. Furthermore, by excluding prior initiations of any of the comparator therapies, there was a single guselkumab cohort. Similarly, after July 13, 2017, specific to each biologic-experienced pairwise comparison: adalimumab, ixekizumab, or secukinumab initiators were excluded if prior to initiation of each respective comparator drug they initiated guselkumab as a biologic-experienced initiation. This exclusion criterion ensured that a patient that had initiated guselkumab prior to initiating the comparator did not contribute to both the treatment and comparator cohorts, thereby violating the independence of observations across treatments assumption.
VARIABLES
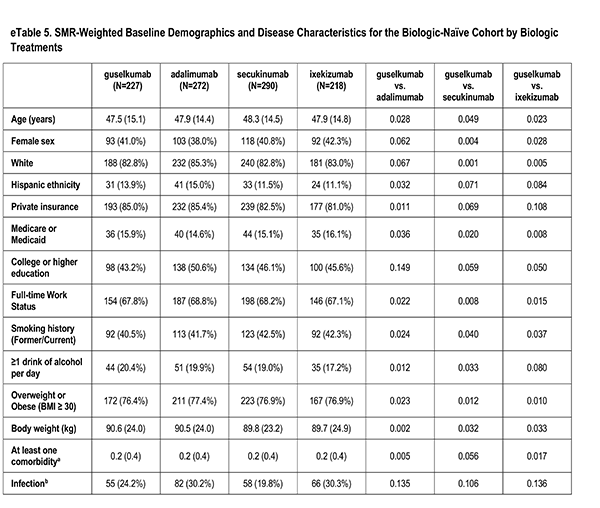
Patient Characteristics
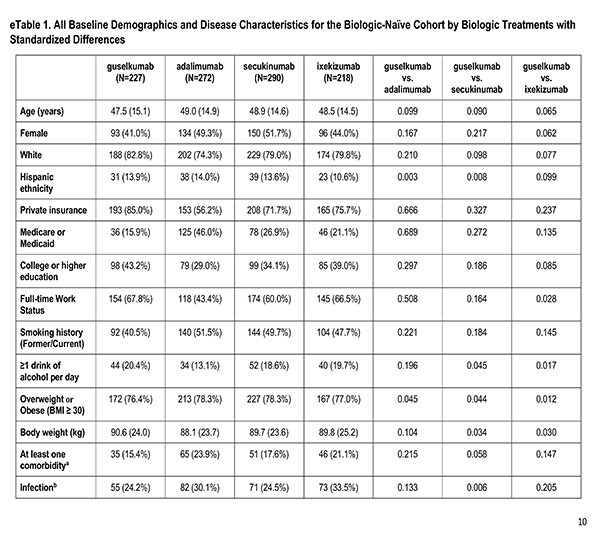
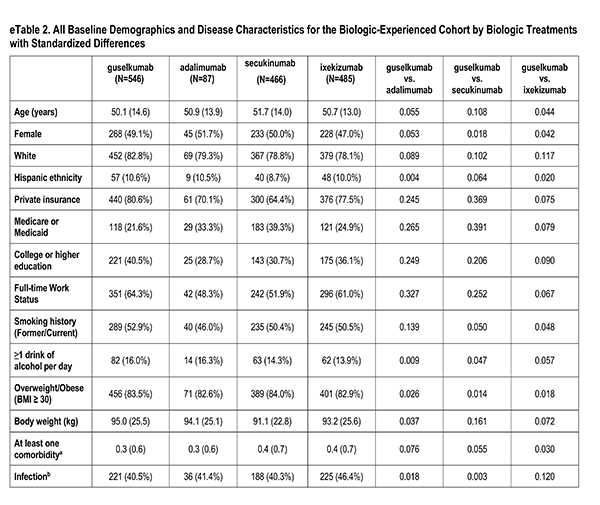
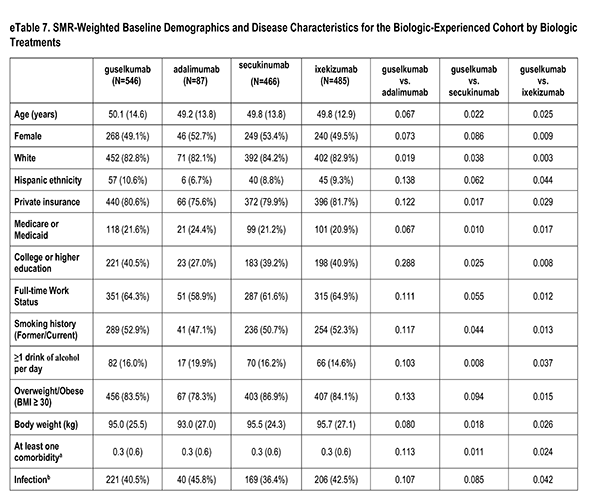
A number of sociodemographic characteristics were measured at index visit including age (years), sex (Male, Female), race (White, Non-White), ethnicity (Hispanic, Non-Hispanic), health insurance (Private, Medicaid or Medicare, Other), education (High School Degree or Less), work status (Full-time, Part-time/Unemployed), smoking history (Never, Former/Current), alcohol use (At least 1 drink per day) and body mass index (Underweight/Normal, Overweight/Obese).
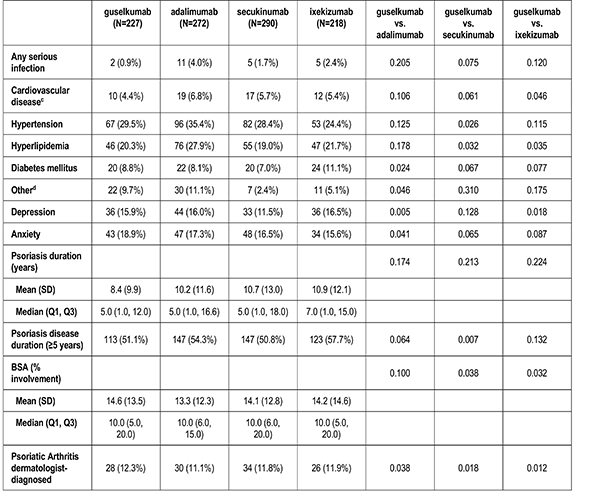
History of Comorbidities
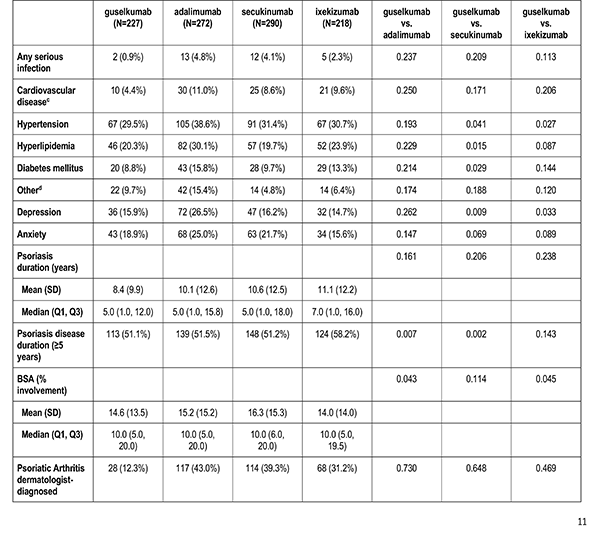
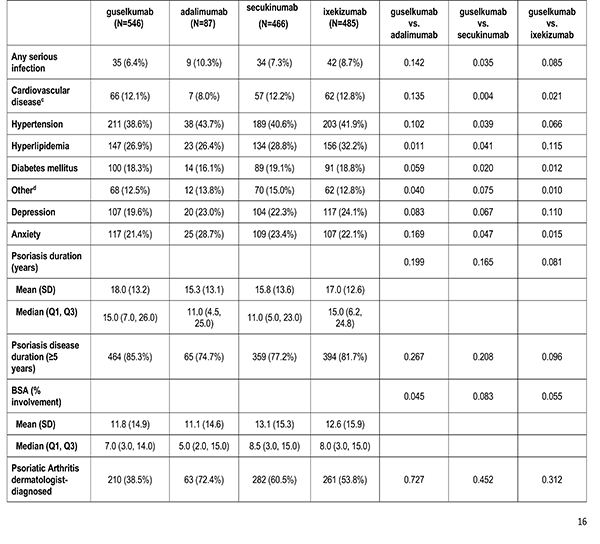
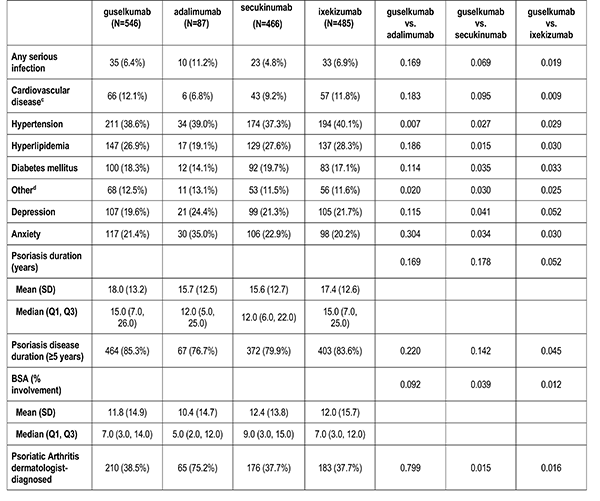
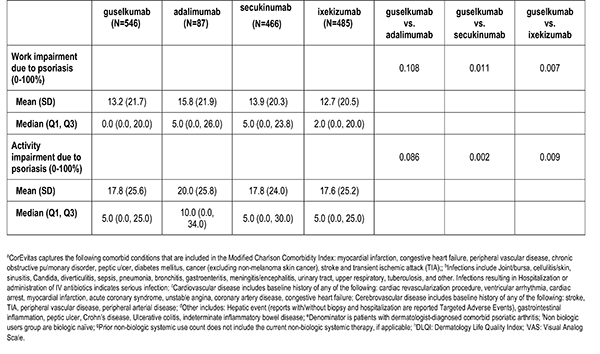
Clinician-reported comorbidities included: infection, serious infection, cardiovascular disease, hypertension, hyperlipidemia, diabetes mellitus, depression, and anxiety.
A number of sociodemographic characteristics were measured at index visit including age (years), sex (Male, Female), race (White, Non-White), ethnicity (Hispanic, Non-Hispanic), health insurance (Private, Medicaid or Medicare, Other), education (High School Degree or Less), work status (Full-time, Part-time/Unemployed), smoking history (Never, Former/Current), alcohol use (At least 1 drink per day) and body mass index (Underweight/Normal, Overweight/Obese).
History of Comorbidities
Clinician-reported comorbidities included: infection, serious infection, cardiovascular disease, hypertension, hyperlipidemia, diabetes mellitus, depression, and anxiety.
Disease Characteristics
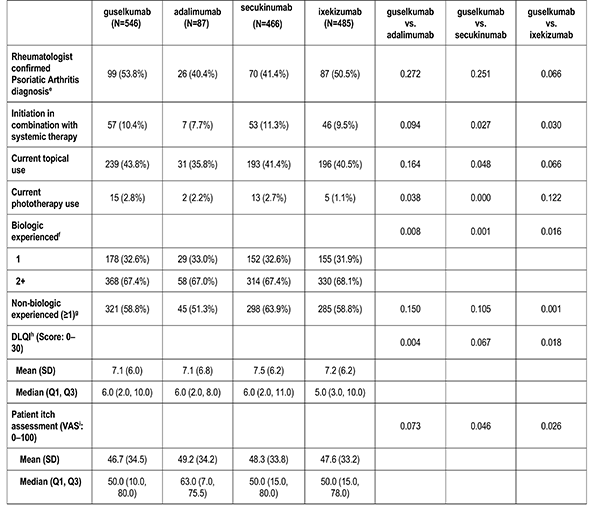
Disease characteristics were collected at the index visit and comprise of psoriasis duration (years), Body Surface Area (BSA), dermatologist diagnosed psoriatic arthritis, and rheumatologist confirmed psoriatic arthritis. Additional characteristics are captured but were not reported in this analysis: Psoriasis Area and Severity Index (PASI), and Investigator Global Assessment (IGA).
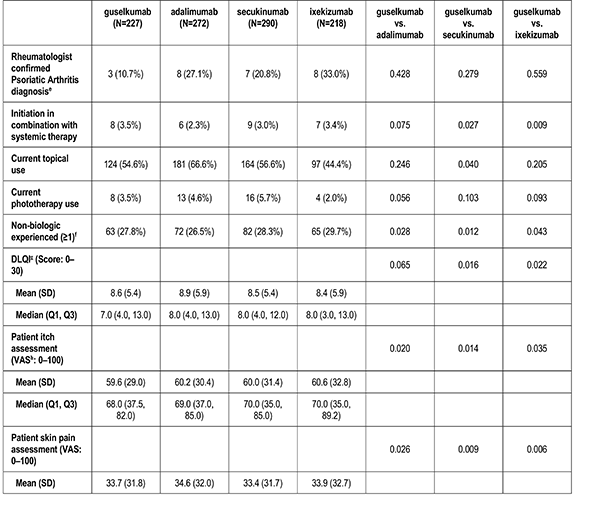
Past and Current Treatment History
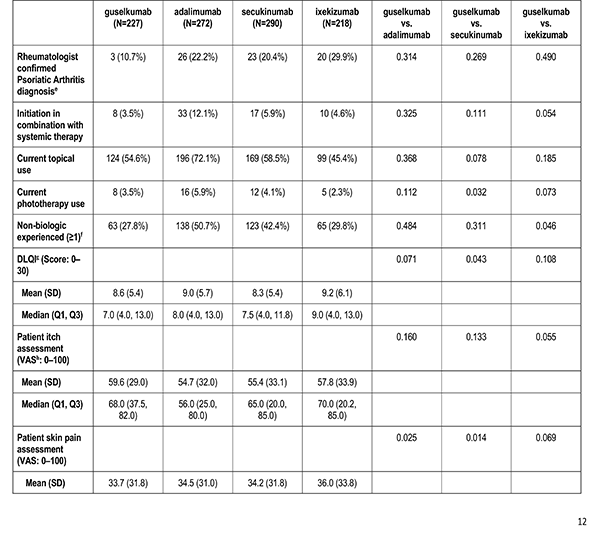
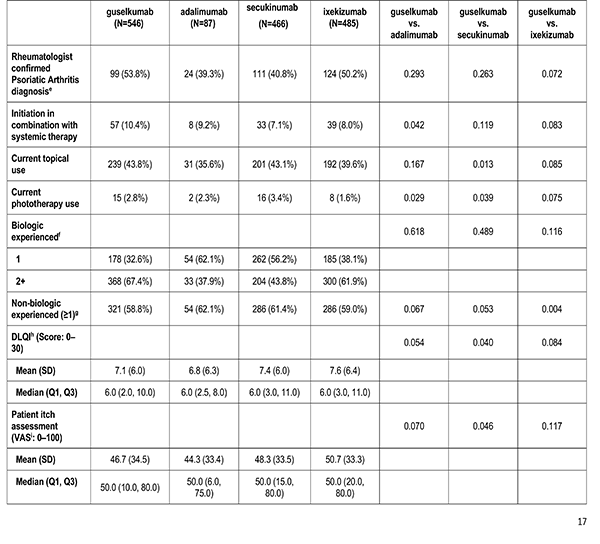
Patient's reported treatment indicators includes current non-biologic systemic use, current topical use, current phototherapy use, biologic therapy experience (1, 2+), and non-biologic therapy experience (greater than or equal to 1).
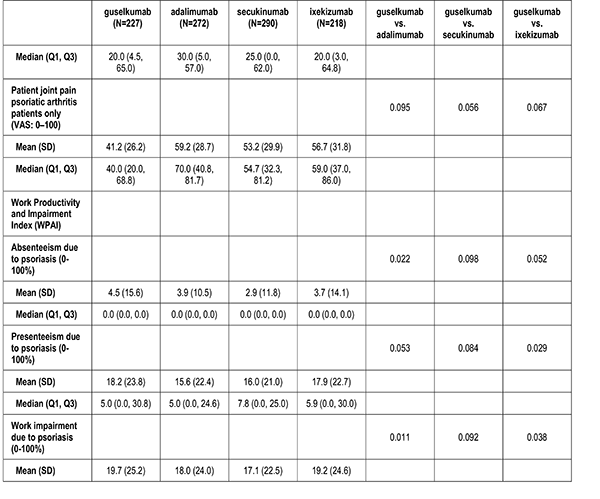
Patient-Reported Outcomes
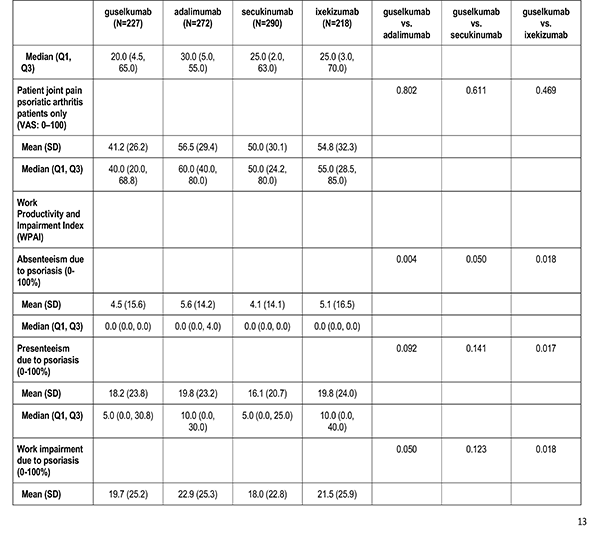
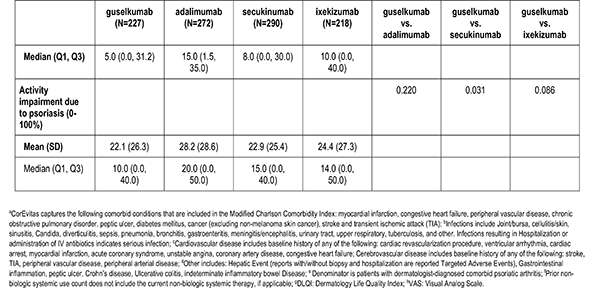
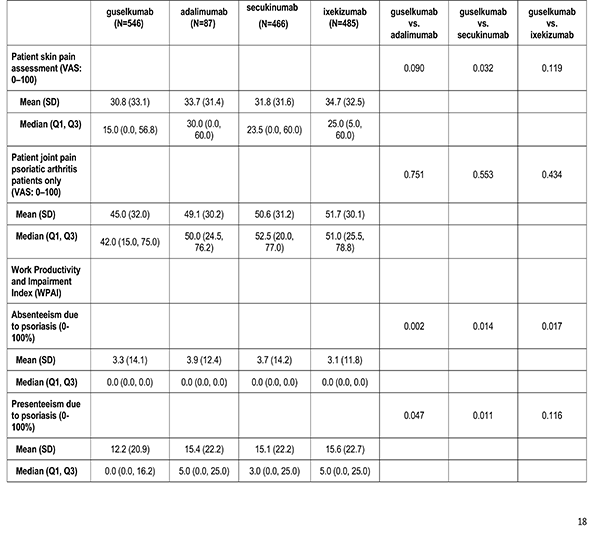
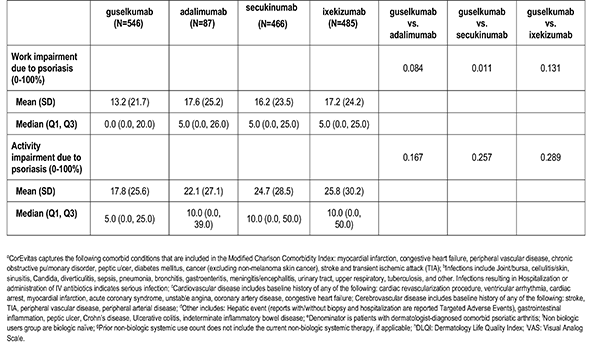
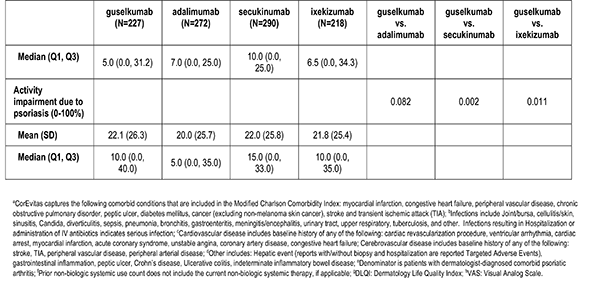
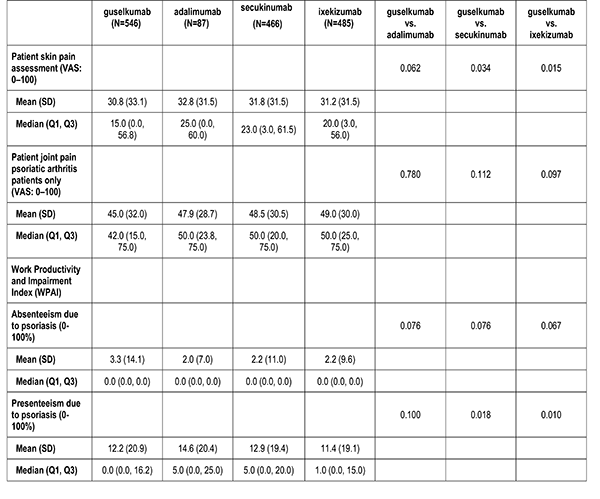
Patient reported outcomes were measured at the index visit. Itch and skin pain were computed on a 100-point Visual Analog Scale (VAS) [0-100]). Work and Productivity Activity Impairment (WPAI) impact were assessed as a percentage for absenteeism, presenteeism, work impairment, and activity impairment. Patients with concomitant psoriatic arthritis also reported joint pain measured on a 100-point VAS scale.
Reasons for Discontinuation
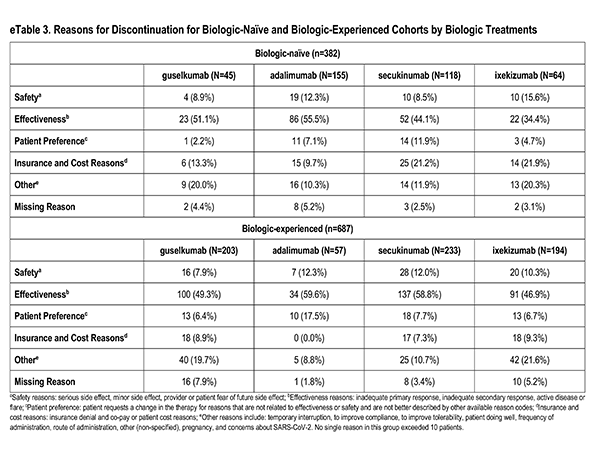
Among patients who discontinued medication, reasons were reported by the physician-investigator and were grouped into the following categories: Safety (serious side effect, minor side effect, provider or patient fear of future side effect): Effectiveness (inadequate primary response, inadequate secondary response, active disease or flare): Patient preference (patient requests a change for reasons not related to effectiveness or safety and not better described by other available reason codes); insurance and cost (insurance denial and co-pay or patient cost reasons); Other (temporary interruption, to improve compliance, to improve tolerability, patient doing well, frequency of administration, route of administration, other (non-specified), pregnancy, concerns about SARS-CoV-2); and missing.
Disease characteristics were collected at the index visit and comprise of psoriasis duration (years), Body Surface Area (BSA), dermatologist diagnosed psoriatic arthritis, and rheumatologist confirmed psoriatic arthritis. Additional characteristics are captured but were not reported in this analysis: Psoriasis Area and Severity Index (PASI), and Investigator Global Assessment (IGA).
Past and Current Treatment History
Patient's reported treatment indicators includes current non-biologic systemic use, current topical use, current phototherapy use, biologic therapy experience (1, 2+), and non-biologic therapy experience (greater than or equal to 1).
Patient-Reported Outcomes
Patient reported outcomes were measured at the index visit. Itch and skin pain were computed on a 100-point Visual Analog Scale (VAS) [0-100]). Work and Productivity Activity Impairment (WPAI) impact were assessed as a percentage for absenteeism, presenteeism, work impairment, and activity impairment. Patients with concomitant psoriatic arthritis also reported joint pain measured on a 100-point VAS scale.
Reasons for Discontinuation
Among patients who discontinued medication, reasons were reported by the physician-investigator and were grouped into the following categories: Safety (serious side effect, minor side effect, provider or patient fear of future side effect): Effectiveness (inadequate primary response, inadequate secondary response, active disease or flare): Patient preference (patient requests a change for reasons not related to effectiveness or safety and not better described by other available reason codes); insurance and cost (insurance denial and co-pay or patient cost reasons); Other (temporary interruption, to improve compliance, to improve tolerability, patient doing well, frequency of administration, route of administration, other (non-specified), pregnancy, concerns about SARS-CoV-2); and missing.
ANALYSIS
Statistical Analysis
Variables included in the final models were as follows: baseline age, sex, race, college education, full time employment, Medicaid or Medicare insurance, smoking history, weight (kg), number of comorbidities, history of anxiety or depression, comorbid dermatologist diagnosed psoriatic arthritis, current non-biologic systemic therapy, non-biologic experience, biologic experience (for bio-experienced comparisons only), total body surface area (BSA) affected by psoriasis , VAS itch, VAS skin pain, dermatology life quality index (DLQI), and the activity impairment domain from the WPAI questionnaire. Weights for each pair of comparisons resulted in the average treatment effect on the treated (ATT) effect estimate with 𝑤! = 1 for initiators of guselkumab and 𝑤! = "! #$"! otherwise, where 𝑤! was the sample weight of the ith participant and 𝑝! was the propensity score for each participant defined as probability that the participant initiates guselkumab, given all covariates, computed with separate pairwise logistic regression models.
Balancing on Disease Activity and Treatment Characteristics for Biologic-Experienced Guselkumab vs. Adalimumab Comparisons
The original study protocol laid out the analysis alternatives to be employed under suboptimal conditions of insufficient overlap in baseline characteristics and inability to achieve balance in confounding variables after respecifications of the SMR model. These alternatives were: 1. overlap weighting, 2. propensity score matching, and 3. inverse probability of treatment weighting (IPTW). After a close assessment of the guselkumab-adalimumab biologic-experienced pair, this pair was found to have very different baseline characteristics and thus required an alternative balancing method; it was also shown that balance could be achieved on either socio-demographic and
Variables included in the final models were as follows: baseline age, sex, race, college education, full time employment, Medicaid or Medicare insurance, smoking history, weight (kg), number of comorbidities, history of anxiety or depression, comorbid dermatologist diagnosed psoriatic arthritis, current non-biologic systemic therapy, non-biologic experience, biologic experience (for bio-experienced comparisons only), total body surface area (BSA) affected by psoriasis , VAS itch, VAS skin pain, dermatology life quality index (DLQI), and the activity impairment domain from the WPAI questionnaire. Weights for each pair of comparisons resulted in the average treatment effect on the treated (ATT) effect estimate with 𝑤! = 1 for initiators of guselkumab and 𝑤! = "! #$"! otherwise, where 𝑤! was the sample weight of the ith participant and 𝑝! was the propensity score for each participant defined as probability that the participant initiates guselkumab, given all covariates, computed with separate pairwise logistic regression models.
Balancing on Disease Activity and Treatment Characteristics for Biologic-Experienced Guselkumab vs. Adalimumab Comparisons
The original study protocol laid out the analysis alternatives to be employed under suboptimal conditions of insufficient overlap in baseline characteristics and inability to achieve balance in confounding variables after respecifications of the SMR model. These alternatives were: 1. overlap weighting, 2. propensity score matching, and 3. inverse probability of treatment weighting (IPTW). After a close assessment of the guselkumab-adalimumab biologic-experienced pair, this pair was found to have very different baseline characteristics and thus required an alternative balancing method; it was also shown that balance could be achieved on either socio-demographic and
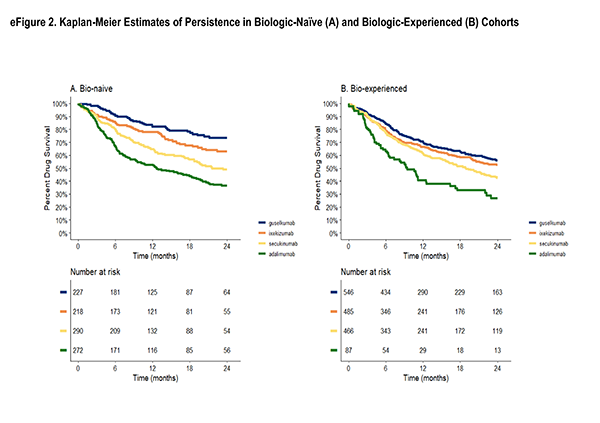
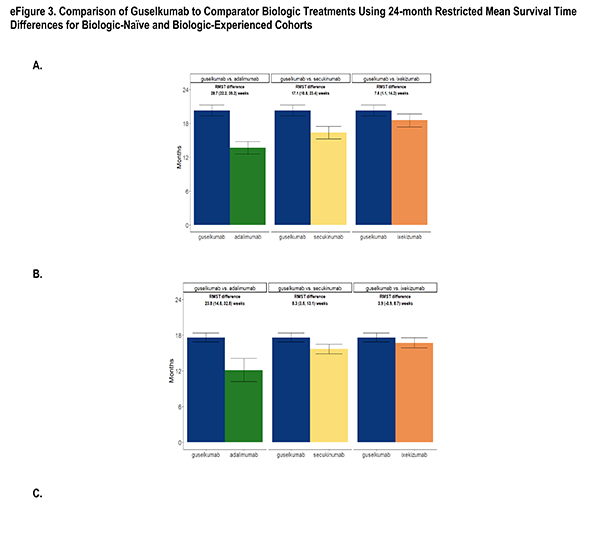
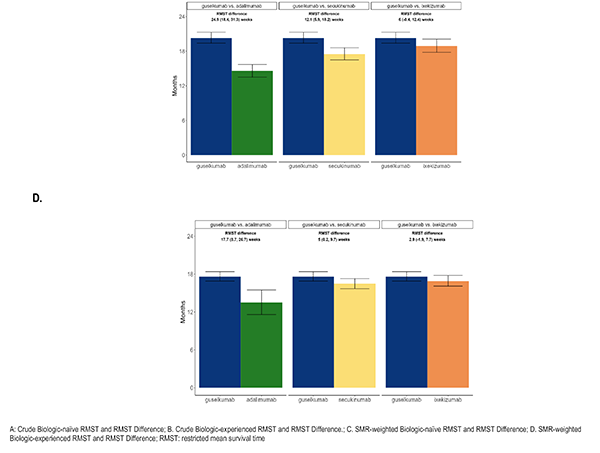
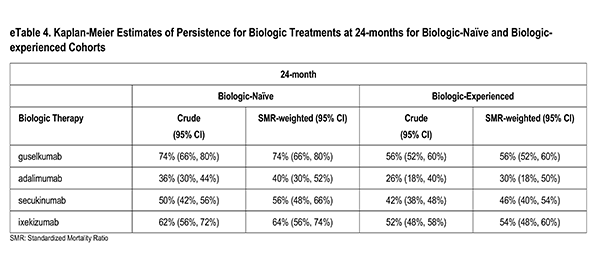
health characteristics or on disease activity and treatment characteristics, but not both. With the desire for the inference to remain in the ATT framework, the optimal solution was to present the results under different balancing methods: one, balancing on disease activity/treatment characteristics1, deemed the primary method and two sensitivity analysis methods: balancing on socio-demographics/health characteristics1 and a truncation method [1]. The truncation method first restricted each sample in the pair to the area of greatest overlap by removing the 10% highest propensity scores in the guselkumab cohort and the 10% lowest propensity scores in the adalimumab cohort, followed by re-fitting of a model with the full set of confounders, which resulted in weighted cohorts on all confounders. The primary method was chosen due to the conservation of the resulting ATT estimates as well as the stronger association of persistence with disease activity/treatment characteristics.
REFERENCES
- Stürmer T, Webster-Clark M, Lund JL, Wyss R, Ellis AR, Lunt M, Rothman KJ, Glynn RJ., "Propensity Score Weighting and Trimming Strategies for Reducing Variance and Bias of Treatment Effect Estimates: A Simulation






