INTRODUCTION
Acne is a common cutaneous inflammatory condition affecting both adolescents and adults worldwide. Acne manifests itself as comedones, papules, pustules, and cysts or nodules on the face, shoulders, chest, and back. Regardless of lesion type or severity of the disease, acne can result in pigmentary alterations and scars.1 Over 60% of post-acne scars did not resolve within 6 months according to a prospective split-face study in 32 patients with moderate facial acne.1 Furthermore, in an international prospective study of 324 patients with acne in Asia and Australia, 32% reported post-inflammatory hyperpigmentation to be more bothersome than acne.2 As such, active disease, as well as the sequelae of acne (post-inflammatory hyperpigmentation and scarring), results in a significant psychosocial burden.3 In this context, early treatment intervention is critical as the incidence of acne scarring increases with increased duration of time before adequate treatment.4
Antibiotics have been a fixture in acne treatment for decades. Their benefit is derived from their anti-inflammatory and antimicrobial properties against Cutibacterium acnes (formerly Propionibacterium acnes), the key pathogenic player in the development of acne.5 With years of antibiotic usage, there is a global unmet need to address the current and future state of antibiotic resistance.6 This review serves to elucidate the pathophysiology of acne focusing on the role of C. acnes, review mechanisms of antibiotic action and resistance, highlight global patterns of C. acnes antibiotic resistance, and discuss the critical utility of implementing benzoyl peroxide (BPO) into a daily acne treatment regimen for overcoming and preventing resistance.
Pathophysiology of Acne Vulgaris and the Role of C. Acnes
Acne arises due to inflammatory dysregulation and dysbiosis of the epidermis and pilosebaceous subunits.7 The 4 pillars of acne pathogenesis are: (1) excess sebum production, (2) follicular epidermal hyperproliferation with plugging, (3) proliferation of C. acnes, and (4) inflammation.8 Follicular plugging further promotes the growth and activity of C. acnes due to the increased anaerobic environment of the pilosebaceous unit. All act in concert with interdependence, with no one component necessarily igniting the disease.
Colonization of C. acnes, a gram-positive lipophilic bacterium that is dominant in sebaceous, lipid-rich areas of skin, is considered a crucial component in the pathogenesis of acne.9,10 C. acnes affects numerous components of skin and inflammatory homeostasis through the following: (1) increasing sebum production, (2) promoting abnormal differentiation and proliferation of keratinocytes, and (3) activating the innate immune system via stimulating pro-inflammatory proteins to be expressed on keratinocyte cell surfaces (eg, Toll-like receptors, TLRs; protease-activated receptors, PARs) and to be secreted into the surrounding environment (eg, matrix metalloproteinases, MMPs).11,12
 C. acnes is classified into 3 phylotypes (I, II, and III) based on gene sequences or biological characteristics (ie, lipase activity).13 These phylotypes are further subdivided into subspecies to delineate their distinct phylogenetic, genomic, and phenotypic characteristics. While there are no quantitative differences in C. acnes of the skin between acne patients and controls, different phylogenetic groups display distinct characteristics that may induce the formation of biofilms and immune responses leading to acne.14 For example, a case-controlled study of 24 patients with severe acne of the face and back found a decrease in C. acnes phylotype diversity with a predominance of phylotype 1A1.15 C. acnes biofilms, which are microbial aggregates embedded in extracellular cellular matrix milieu that allow for inter-microbial communication and protection from host surveillance mechanisms, are also thought to act as an adhesive within sebum thereby increasing the cohesiveness of keratinocytes in the pilosebaceous unit leading to the formation of microcomedones.16 Jahns et al corroborate this notion in their case-control study, which demonstrated that more frequent biofilm formation in sebaceous follicles was found in facial biopsy samples of acne patients compared to control samples.17 In addition to comedone formation, C. acnes is considered etiological to inflammatory acne lesions due to the interaction of C. acnes with numerous biological targets as previously noted.18 Interestingly, acne-associated C. acnes phylotypes induce higher concentrations of interferon-gamma (IFN-y) and interleukin (IL)-17 suggesting activation of both Th1 and Th17 pathways possibly due to expression of different antigenic components compared to phylotypes associated with normal skin, which are associated with induction of higher levels of IL-10.13,19
C. acnes is classified into 3 phylotypes (I, II, and III) based on gene sequences or biological characteristics (ie, lipase activity).13 These phylotypes are further subdivided into subspecies to delineate their distinct phylogenetic, genomic, and phenotypic characteristics. While there are no quantitative differences in C. acnes of the skin between acne patients and controls, different phylogenetic groups display distinct characteristics that may induce the formation of biofilms and immune responses leading to acne.14 For example, a case-controlled study of 24 patients with severe acne of the face and back found a decrease in C. acnes phylotype diversity with a predominance of phylotype 1A1.15 C. acnes biofilms, which are microbial aggregates embedded in extracellular cellular matrix milieu that allow for inter-microbial communication and protection from host surveillance mechanisms, are also thought to act as an adhesive within sebum thereby increasing the cohesiveness of keratinocytes in the pilosebaceous unit leading to the formation of microcomedones.16 Jahns et al corroborate this notion in their case-control study, which demonstrated that more frequent biofilm formation in sebaceous follicles was found in facial biopsy samples of acne patients compared to control samples.17 In addition to comedone formation, C. acnes is considered etiological to inflammatory acne lesions due to the interaction of C. acnes with numerous biological targets as previously noted.18 Interestingly, acne-associated C. acnes phylotypes induce higher concentrations of interferon-gamma (IFN-y) and interleukin (IL)-17 suggesting activation of both Th1 and Th17 pathways possibly due to expression of different antigenic components compared to phylotypes associated with normal skin, which are associated with induction of higher levels of IL-10.13,19
To date, there is no case of acne without C. acnes, and therapeutically induced reduction of C. acnes population directly or indirectly via antibiotics or retinoids (through suppression of sebum production), respectively, reduces inflammation.9 Thus, targeting C. acnes is a mainstay in acne treatment for all patients regardless of skin type, disease severity, and lesion type.
Current Paradigm in Acne Treatment
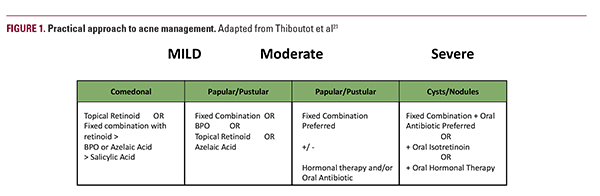
Current treatments focus on simultaneously targeting multiple aspects of acne pathophysiology: reducing sebum production, normalizing keratinization, killing C. acnes, and reducing inflammation.20 According to a 2018 Delphi international consensus as well as guidelines from the European Dermatology Forum and American Academy of Dermatology, topical retinoids are the first line in mild or moderate disease as they affect all components and may be used as monotherapy or in combination with other therapeutics (Figure 1).21-23 Addition of topical antibiotics to target C. acnes is also advised in combination with a retinoid but not as monotherapy given the worldwide problem of antibiotic resistance.24-27 Among antimicrobials, BPO is the preferred topical agent given its mechanism of action as a strong oxidative agent with potent bactericidal activity while preventing the development of antibiotic resistance.23 For patients with severe and moderately severe acne recalcitrant or intolerant to topical combination therapy with topical retinoid and BPO, the addition of oral antibiotic, oral isotretinoin, or hormonal therapy is recommended by the guidelines (Figure 1). While the AAD guidelines recommend oral antibiotic use for 3 to 4 months, recent studies found that approximately 20% of patients continuously used them for ≥ 6 months.28,29
The utilization of antibiotics in dermatology, particularly for the treatment of acne, has come under intense scrutiny. According to the Scientific Panel on Antibiotic Use in Dermatology (SPAUD), which operates under the purview of the American Acne and Rosacea Society, dermatologists prescribe ~8.2 million oral antibiotic prescriptions annually.30 Given the high prevalence of acne worldwide, the use of antibiotics must be judicious as there is a massive selective pressure of these treatments on both pathogenic and nonpathogenic microbes that lead to alterations in the microbiome and selection of antibiotic-resistant organisms. Not only do antibiotic prescribing patterns affect resistance patterns of C. acnes relevant for acne treatment but also that of numerous other organisms and their sites of colonization, which have far-reaching implications for infectious diseases of both the skin and viscera.30 For example,
a 3-fold greater incidence of oropharyngeal colonization with Streptococcus pyogenes (with 85% exhibiting resistance to at least one tetracycline antibiotic) was found in patients using oral and/or topical antibiotics for at least 3 months compared to control patients without antibiotics for at least 6 months.31 A large retrospective cohort analysis of over 118,000 patients found a 2.15-fold greater risk of developing an upper respiratory tract infection in those treated with oral and/or topical antibiotics.32 In addition, facial application of erythromycin 2% gel for 12 weeks resulted in a greater amount of S. aureus nasal carriage as well as erythromycin-resistant coagulase-negative staphylococci on the face and at remote sites such as the back and anterior nares.33 These erythromycin-resistant bacteria persisted over at least 4 weeks despite discontinuation of antibiotic treatment.
The implications of antibiotic use in a global context cannot be understated. With respect to acne, understanding antibiotic resistance patterns of C. acnes is critical as appropriate antibiotic usage is a lynchpin in the acne treatment algorithm.
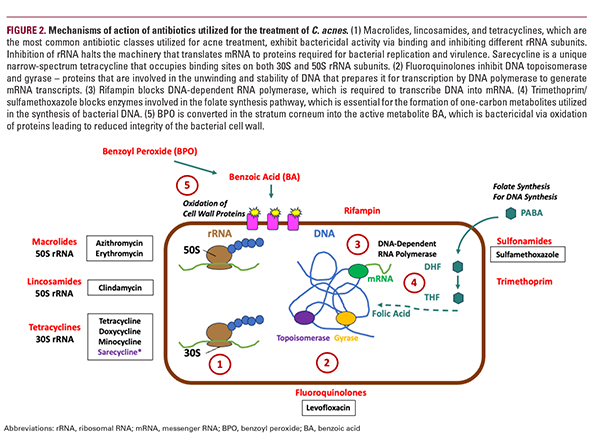
Current Antibiotics Utilized in the Treatment of Acne Numerous antibiotics of different classes have been utilized to target C. acnes (Figure 2). The most commonly prescribed oral antibiotics in the United States from 1993 to 2016 according to a National Ambulatory Medical Care Survey (NAMCS) are minocycline, doxycycline, and clindamycin.34 The major classes of antibiotics employed for treating acne include the following: (1) tetracyclines, (2) lincosamides, and (3) macrolides. All 3 classes target ribosomal RNA (rRNA) — the machinery by which bacterial proteins are translated from messenger RNA (mRNA) into amino acids and arranged into functioning peptides. They, however, target different components of this machinery; macrolides and lincosamides bind and inhibit the 50S larger ribosomal subunit albeit at different positions, whereas tetracyclines inhibit the 30S smaller ribosomal subunit (Figure 2). Given their established safety and efficacy, tetracyclines are the preferred first-line treatment option for acne.35 Commonly used oral antibiotics in the tetracycline class include tetracycline, doxycycline, minocycline, and,
 more recently, sarecycline.36 Sarecycline unexpectedly binds simultaneously to both the 30S and 50S subunits in C. acnes, which lends to its narrow-spectrum activity.37 In addition to the tetracyclines, clindamycin is the canonical lincosamide utilized in acne treatment.38 From the macrolide group, azithromycin and erythromycin are the most utilized.35
more recently, sarecycline.36 Sarecycline unexpectedly binds simultaneously to both the 30S and 50S subunits in C. acnes, which lends to its narrow-spectrum activity.37 In addition to the tetracyclines, clindamycin is the canonical lincosamide utilized in acne treatment.38 From the macrolide group, azithromycin and erythromycin are the most utilized.35
Although not considered a typical antibiotic, BPO exhibits a unique mechanism of action whereby it is converted to benzoic acid (BA) in the stratum corneum, which then non-specifically oxidizes bacterial cell wall proteins, thus exerting bactericidal activity.39 The importance of BPO and this mechanism of action is discussed in detail in a later section.
Other antibiotics also used for acne treatment but at much lower rates include fluoroquinolones and sulfonamides (Figure 2). Fluoroquinolones, such as levofloxacin, inhibit DNA topoisomerase and gyrase, which are involved in bacterial DNA unwinding and stability in preparation for transcription of DNA into mRNA.40,41 Sulfonamides, such as sulfamethoxazole, block the formation of dihydrofolate in the folate synthesis pathway, which is a critical metabolite in microbial DNA synthesis. A combination of sulfamethoxazole with trimethoprim is often employed as trimethoprim also inhibits the formation of tetrahydrofolate in the folate synthesis pathway.42 Rifampin, an antimycobacterial, binds to DNA-dependent RNA polymerase to inhibit the transcription of bacterial DNA. While it is not yet used for acne vulgaris, it has utility against C. acnes biofilm in a foreign body infection model.43 It, therefore, may theoretically be effective for the treatment of acne.
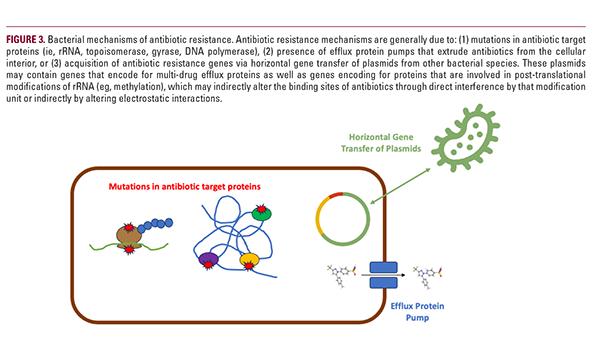
C. Acnes Mechanisms of Acquiring Antibiotic Resistance Targeting C. acnes is central to acne treatment success. As antibiotic agents have been utilized for billions of patients worldwide for many decades, antibiotic-resistant C. acnes strains have invariably emerged and now pose challenges for current and future acne patients. Before the turn of the 21st century, C. acnes resistance rose over 40% from 20% in 1978 to 62% in 1996.44 Furthermore, there are also instances of antibiotic-resistant C. acnes in patients who have never undergone antibiotic treatment.45 Thus, it is important to understand the mechanisms behind how C. acnes acquires resistance (Figure 3).
Mutations in genes encoding ribosomal RNA are frequently found in C. acnes strains isolated from acne patients exhibiting macrolide resistance.46 23S rRNA, found within the 50S subunit, is the microbial target of macrolides (ie, clindamycin), and mutations at the binding site of these antibiotics result in their inability to bind and inhibit its function. This resistance is thought to be caused by long-term, low-concentration exposure of macrolides thereby providing a low-level selection
pressure.47 Similarly, mutations in 16S rRNA (found within the 30S subunit), as well as DNA gyrase and topoisomerase genes (gyrA and parC, respectively), explain resistance to tetracycline and fluoroquinolones, respectively.48,49
Efflux protein pumps are another mechanism of resistance where bacteria pump out antibiotics from the cellular interior to the exterior.50,51 Notably, efflux pumps can selectively extrude a distinct class of antibiotics or can pump out several classes leading to multi-drug resistant bacteria.52 Genes encoding efflux protein pumps could be intrinsic to the bacteria or acquired through horizontal gene transfer from other bacteria.53
C. acnes antibiotic resistance may also be due to acquisition of exogenous resistance genes, which are transmitted between bacteria, also known as horizontal gene transfer.54,55 These genes include erm(x) and erm(50), which are found on plasmids that are independent from the C. acnes genome, and function to methylate 23S rRNA.56 Multi-drug resistant strains more commonly harbor plasmids containing these genes, which are transmitted not only between Cutibacterium strains but also between C. acnes and other species, including the major skin commensal bacterium Staphylococcus epidermidis.57 This phenomenon of plasmid transfer explains why previously antibiotic-naïve patients with acne harbor resistant C. acnes strains.
Antibiotic Resistance Patterns – A Global Outlook
Antibiotic resistance patterns of C. acnes vary among different world regions due to prescribing patterns.14 In the United States, resistance rates ordered from greatest to least are for erythromycin followed by clindamycin and tetracycline.30 Rates of resistance to any antibiotic ranged from 50.8% to 93.6% across numerous countries, including the UK, Spain, Italy, Greece, Sweden, and Hungary.58 In Japan, resistance rates were documented to be 2.5 times higher in 2020 than in 2013.45 Overall, many countries have reported that antibiotic resistance is present in over 50% of C. acnes strains.59
The antibiotics with the greatest overall resistance rates were the macrolides and lincosamides. In France and Spain, approximately 70% and 91% of strains exhibited resistance to clindamycin and erythromycin.60 Japan exhibited resistance rates of approximately 19% and 23% to clindamycin and erythromycin, respectively; while Bangkok, Thailand, had resistance rates of over 70% for both.61,62 In Hong Kong, China, resistance rates were 53.5% and 20.9% for clindamycin and erythromycin, respectively.63 Azithromycin resistance, however, has been scarcely studied. Nonetheless, a study in Mexico reported 82% resistance and a study in India reported 100% resistance.64,65 High resistance rates to azithromycin were also found in China.66
The tetracycline class also experienced significant resistance rates albeit less than the macrolides and lincosamides. Resistance rates to tetracycline in France and Spain ranged from 0% to 26.4%.58 Hong Kong similarly had 16.3% resistance to tetracyclines.63 For doxycycline and minocycline, resistance rates in Japan were 4.3% and 0%, respectively.61 In Thailand, resistance rates were 51.7% and 51.1% for tetracycline and doxycycline, respectively.62
With respect to trimethoprim/sulfamethoxazole, studies in Jordan, Thailand, and Mexico reported resistance rates of C. acnes isolates to be 31%, 100%, and 68%, respectively.62,64,67 For fluoroquinolones, resistance rates were 15% for levofloxacin in Jordan and 6.3% for moxifloxacin in China.67,68 While considering the aforementioned reported rates of resistance, it is important to note that there is significant variation in sampling (eg, superficial swabs, biopsies) and total number of patients per country.
Utilization of BPO for Antibiotic Stewardship in Acne
BPO is available in numerous over-the-counter and prescription formulations such as washes, lotions, and gels. It is converted to BA once it penetrates the stratum corneum after topical application.69 During this conversion, highly reactive oxygen species are generated, which oxidize bacterial proteins thereby damaging cell walls.39,70 As such, BPO is effective against gram-positive bacteria and fungi but less so against gram-negative bacteria due to the presence of outer membranes.39 In addition to its antimicrobial effects, BPO also increases the rate of epithelial cell turnover and desquamation thereby exhibiting keratolytic and comedolytic effects.70
The mechanism of BPO bactericidal activity is unique among antibiotics in that it is not targeted toward particular bacterial machinery such as rRNA but is rather a generalized approach aimed at dismantling the bacterial cell wall. BPO can therefore be used against a wide variety of bacteria despite their resistance patterns while also minimizing the risk of selecting resistant bacteria.71 No resistance has been reported with BPO when used in acne treatment to date.23 As such, BPO is considered first-line as topical monotherapy or in combination with oral antibiotics in the AAD guidelines to reduce the risk of resistance development.21 This is exemplified by a study that demonstrated that daily application of the topical combination drug 2.5% BPO/0.1% adapalene for 4 weeks resulted in a 97% reduction of antibiotic-susceptible and antibiotic-resistant C. acnes strains with some resistant strains being eliminated in some patients.72
Combination Topical Drugs Incorporating BPO for Acne Treatment
While BPO has been incorporated in various combination
regiments, there have been some limitations. These have generally included tolerability and oxidation.73,74 Traditional BPO formulations and higher concentrations of BPO tend to cause skin irritation such as dryness, peeling, erythema, and stinging/burning. This has been addressed with the development of novel vehicles and colloidal delivery systems.75-77 BPO is also highly oxidative, which leads to potential bleaching of clothing and hair. This oxidative property also causes degradation of tretinoin, thereby reducing treatment efficacy and classically precluding their combined application.78 Microencapsulation and micronization technologies now allow for the combination of BPO and tretinoin while reducing oxidation.79
Notwithstanding these limitations, BPO has been incorporated with retinoids and antibiotics resulting in a plethora of dyad combination topical products that are synergistically effective in treating acne while minimizing antibiotic resistance. Topical antibiotic combination treatments include BPO with either clindamycin or erythromycin at varying concentrations.80 BPO 5%/clindamycin phosphate 1% gel,81 BPO 3.75%/clindamycin phosphate 1.2% gel,82 and BPO 2.5% / clindamycin phosphate 1.2% gel83 are the currently approved BPO/clindamycin combinations for patients 12 years and older. Microencapsulated tretinoin 0.1%/BPO 0.3% cream was recently approved in 2021 for the treatment of acne in patients ≥ 9 years of age.84 BPO 2.5% gel in combination with different concentrations of adapalene (0.1% and 0.3%) has also been approved for acne patients aged ≥ 9 and 12 years, respectively.85
A novel first-in-class, fixed-dose, triple combination topical gel (IDP-126) containing clindamycin phosphate 1.2%, BPO 3.1% and adapalene 0.15% in a polymeric mesh gel with micronized particles of BPO and adapalene was recently studied in a 12-week phase 2 double-blind, parallel-group, randomized and vehicle-controlled clinical trial in patients aged ≥ 9 years with moderate-to-severe acne.86 Once-daily topical application of IDP-126 achieved treatment success (>50% of participants) over vehicle at week 12 with over 70% reductions in inflammatory and noninflammatory lesions.86 Furthermore, treatment success rates appeared to be greater (synergistic) than the expected additive effect of each component as the rates were 1.7-1.8 times greater than with the component dyads. Similar treatment success was demonstrated in 2 identically designed, 12-week phase 3 pivotal trials.87 Furthermore, a head-to-head study comparing the efficacy of IDP-126 to Epiduo® Forte (BPO 2.5%/adapalene 0.3% gel) is currently underway (NCT04892706).88 Given these results and the polymeric mesh technology that now allows for BPO to be combined with previously incompatible actives such as tretinoin due to oxidation, triple combination IDP-126 offers a potential new once-daily tolerable “monotherapy” that may exhibit synergistic efficacy by simultaneously affecting multiple pathophysiologic factors giving rise to acne while also minimizing the development of C. acnes antibiotic resistance strains.
CONCLUSION
Targeting C. acnes is a critical component in all international guidelines for the treatment of acne.21 While this is done with the use of topical and oral antibiotics, it has been found that antibiotic treatment is being prescribed well over the recommended limit of 3 months, thereby leading to a selection pressure for antibiotic resistance.28 The rise in C. acnes antibiotic resistance is a global phenomenon given the ubiquitous use of antibiotics in billions of patients with acne. Antibiotic stewardship is now more important than ever, not only because the effectiveness of current antibiotic regimens for acne will decline but also because other pathologies due to virulent C. acnes, such as prosthetic joint infections, may become more prevalent and difficult to treat.9 Vaccines against C. acnes are also being studied in an effort to circumvent the use of antibiotics.89-91
BPO, an age-old topical medication with a unique antimicrobial mechanism of action where its metabolite BA oxidizes bacterial proteins thereby destroying cell walls, emerges as a necessary agent to combat antibiotic resistance. This seemingly non-targeted yet elegant mechanism has proven effective in treating both non-resistant and antibiotic resistant C. acnes strains.71 Furthermore, use of BPO in acne treatment has resulted in preventing C. acnes resistance.23 Advances in vehicle and encapsulation technologies have also allowed BPO to be elegantly formulated with other antibiotics and retinoids such as tretinoin despite its oxidative capacity. This is evident with numerous topical BPO dyads already approved for the treatment of acne and now with the emergence of IDP-126, the first-in-class topical triple-combination gel containing clindamycin phosphate 1.2%, BPO 3.1%, and adapalene 0.15%. Taking together the data highlighting the efficacy of BPO on all strains of C. acnes while minimizing the development of resistance, implementation of BPO should be considered in every patient with acne in our era of antibiotic overuse.
DISCLOSURES
Leon Kircik MD has served as either an investigator, consultant, speaker or advisory board member for Allergan, Allmirall, Biofrontera, Galderma, L'Oreal, Mayne Pharma, Ortho Dermatologics, and SUN Pharma. Naiem T. Issa has received funding from the following entities either as a speaker, consultant, advisor, or investigator from Bristol Myers Squibb, Castle Biosciences, Dermavant Sciences, DermTech, Galderma, LEO Pharma, Lilly, National Eczema Association, Ortho Dermatologics, Pfizer, RBC Consultants, Verrica Pharmaceuticals, and WebMD.
REFERENCES
- Tan J, Bourdès V, Bissonnette R, et al. Prospective study of pathogenesis of atrophic acne scars and role of macular erythema. J Drugs Dermatol. 2017;16(6):566-572.
- Abad-Casintahan F, Chow SKW, Goh CL, et al. Frequency and characteristics of acne-related post-inflammatory hyperpigmentation. J Dermatol. 2016;43(7):826-828. doi:10.1111/1346-8138.13263
- Zhou C, Vempati A, Tam C, et al. Beyond the surface: A deeper look at the psychosocial impacts of acne scarring. Clin Cosmet Investig Dermatol. 2023;16:731-738. doi:10.2147/CCID.S406235
- Layton AM, Henderson CA, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19(4):303-308. doi:10.1111/j.1365-2230.1994.tb01200.x
- Rusu A, Buta EL. The development of third-generation tetracycline antibiotics and new perspectives. Pharmaceutics. 2021;13(12):2085. doi:10.3390/pharmaceutics13122085
- Dessinioti C, Katsambas A. Antibiotics and antimicrobial resistance in acne: Epidemiological trends and clinical practice considerations. Yale J Biol Med. 2022;95(4):429-443.
- Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet. 2012;379(9813):361-372. doi:10.1016/s0140- 6736(11)60321-8
- Zouboulis CC, Eady A, Philpott M, et al. What is the pathogenesis of acne? Exp Dermatol. 2005;14(2):143- 152. doi:10.1111/j.0906-6705.2005.0285a.x
- Brüggemann H, Salar-Vidal L, Gollnick HPM, et al. A Janus-Faced Bacterium: Host-Beneficial and -Detrimental Roles of Cutibacterium acnes. Front Microbiol. 2021;12:673845. doi:10.3389/fmicb.2021.673845
- Scholz CFP, Kilian M. The natural history of cutaneous propionibacteria, and reclassification of selected species within the genus Propionibacterium to the proposed novel genera Acidipropionibacterium gen. nov., Cutibacterium gen. nov. and Pseudopropionibacterium gen. nov. Int J Syst Evol Microbiol. 2016;66(11):4422- 4432. doi:10.1099/ijsem.0.001367
- Beylot C, Auffret N, Poli F, et al. Propionibacterium acnes: an update on its role in the pathogenesis of acne. J Eur Acad Dermatol Venereol. 2014;28(3):271-278. doi:10.1111/jdv.12224
- Del Rosso JQ, Kircik LH. The sequence of inflammation, relevant biomarkers, and the pathogenesis of acne vulgaris: what does recent research show and what does it mean to the clinician? J Drugs Dermatol. 2013;12(8 Suppl):s109-15.
- Yu Y, Champer J, Agak GW, et al. Different Propionibacterium acnes phylotypes induce distinct immune responses and express unique surface and secreted proteomes. J Invest Dermatol. 2016;136(11):2221-2228. doi:10.1016/j.jid.2016.06.615
- Platsidaki E, Dessinioti C. Recent Advances in Understanding Propionibacterium Acnes (Cutibacterium Acnes) in Acne. F1000Res.; 2018. doi:10.12688/f1000research.15659.1
- Dagnelie M, Corvec S, Saint-Jean M, et al. Decrease in diversity of Propionibacterium acnes phylotypes in patients with severe acne on the back. Acta Derm Venereol. 2018;98(2):262-267. doi:10.2340/00015555-2847
- Burkhart CG, Burkhart CN. Expanding the microcomedone theory and acne therapeutics: Propionibacterium acnes biofilm produces biological glue that holds corneocytes together to form plug. J Am Acad Dermatol. 2007;57(4):722-724. doi:10.1016/j.jaad.2007.05.013
- Jahns AC, Lundskog B, Ganceviciene R, et al. An increased incidence of Propionibacterium acnes biofilms in acne vulgaris: a case-control study: Increased incidence of P. acnes biofilms in acne vulgaris. Br J Dermatol. 2012;167(1):50-58. doi:10.1111/j.1365-2133.2012.10897.x
- Dessinioti C, Katsambas AD. The role of Propionibacterium acnes in acne pathogenesis: facts and controversies. Clin Dermatol. 2010;28(1):2-7. doi:10.1016/j.clindermatol.2009.03.012
- Agak GW, Kao S, Ouyang K, et al. Phenotype and antimicrobial activity of Th17 cells induced by Propionibacterium acnes strains associated with healthy and acne skin. J Invest Dermatol. 2018;138(2):316-324. doi:10.1016/j.jid.2017.07.842
- Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(5 Suppl):S1-50. doi:10.1016/j. jaad.2009.01.019
- Thiboutot DM, Dréno B, Abanmi A, et al. Practical management of acne for clinicians: An international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2018;78(2 Suppl 1):S1- S23.e1. doi:10.1016/j.jaad.2017.09.078
- Nast A, Dréno B, Bettoli V, et al. European evidence-based (S3) guideline for the treatment of acne - update 2016 - short version. J Eur Acad Dermatol Venereol. 2016;30(8):1261-1268. doi:10.1111/jdv.13776
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945-73.e33. doi:10.1016/j.jaad.2015.12.037
- Dreno B, Thiboutot D, Gollnick H, et al. Antibiotic stewardship in dermatology: limiting antibiotic use in acne. Eur J Dermatol. 2014;24(3):330-334. doi:10.1684/ejd.2014.2309
- Thiboutot D, Dreno B, Gollnick H, et al. A call to limit antibiotic use in acne. J Drugs Dermatol. 2013;12(12):1331-1332.
- Simonart T, Dramaix M. Treatment of acne with topical antibiotics: lessons from clinical studies. Br J Dermatol. 2005;153(2):395-403. doi:10.1111/j.1365-2133.2005.06614.x
- Tzellos T, Zampeli V, Makrantonaki E, et al. Treating acne with antibiotic-resistant bacterial colonization. Expert Opin Pharmacother. 2011;12(8):1233-1247. doi:10.1517/14656566.2011.553192
- Grada A, Armstrong A, Bunick C, et al. Trends in oral antibiotic use for acne treatment: A retrospective, population-based study in the United States, 2014 to 2016. J Drugs Dermatol. 2023;22(3):265-270. doi:10.36849/JDD.7345
- Lee YH, Liu G, Thiboutot DM, et al. A retrospective analysis of the duration of oral antibiotic therapy for the treatment of acne among adolescents: investigating practice gaps and potential cost-savings. J Am Acad Dermatol. 2014;71(1):70-76. doi:10.1016/j.jaad.2014.02.031
- Del Rosso JQ, Webster GF, Rosen T, et al. Status report from the scientific panel on antibiotic use in dermatology of the American acne and Rosacea society: Part 1: Antibiotic prescribing patterns, sources of antibiotic exposure, antibiotic consumption and emergence of antibiotic resistance, impact of alterations in antibiotic prescribing, and clinical sequelae of antibiotic use. J Clin Aesthet Dermatol. 2016;9(4):18-24.
- Levy RM, Huang EY, Roling D, et al. Effect of antibiotics on the oropharyngeal flora in patients with acne. Arch Dermatol. 2003;139(4):467-471. doi:10.1001/archderm.139.4.467
- Margolis DJ, Bowe WP, Hoffstad O, et al. Antibiotic treatment of acne may be associated with upper respiratory tract infections. Arch Dermatol. 2005;141(9):1132-1136. doi:10.1001/archderm.141.9.1132
- Mills O Jr, Thornsberry C, Cardin CW, et al. Bacterial resistance and therapeutic outcome following three months of topical acne therapy with 2% erythromycin gel versus its vehicle. Acta Derm Venereol. 2002;82(4):260-265. doi:10.1080/000155502320323216
- Perche PO, Peck GM, Robinson L, et al. Prescribing trends for acne vulgaris visits in the United States. Antibiotics (Basel). 2023;12(2):269. doi:10.3390/antibiotics12020269
- Baldwin H. Oral antibiotic treatment options for acne vulgaris. J Clin Aesthet Dermatol. 2020;13(9):26-32.
- Tao RE, Prajapati S, Pixley JN, et al. Oral tetracycline-class drugs in dermatology: Impact of food intake on absorption and efficacy. Antibiotics (Basel). 2023;12(7). doi:10.3390/antibiotics12071152
- Lomakin IB, Devarkar SC, Patel S, et al. Sarecycline inhibits protein translation in Cutibacterium acnes 70S ribosome using a two-site mechanism. Nucleic Acids Res. 2023;51(6):2915-2930. doi:10.1093/nar/gkad103
- Guay DRP. Topical clindamycin in the management of acne vulgaris. Expert Opin Pharmacother. 2007;8(15):2625-2664. doi:10.1517/14656566.8.15.2625
- Okamoto K, Kanayama S, Ikeda F, et al. Broad spectrum in vitro microbicidal activity of benzoyl peroxide against microorganisms related to cutaneous diseases. J Dermatol. 2021;48(4):551-555. doi:10.1111/1346- 8138.15739
- Kawada A, Aragane Y, Tezuka T. Levofloxacin is effective for inflammatory acne and achieves high levels in the lesions: an open study. Dermatology. 2002;204(4):301-302. doi:10.1159/000063365
- Kawada A, Wada T, Oiso N. Clinical effectiveness of once-daily levofloxacin for inflammatory acne with high concentrations in the lesions: Letters to the Editor. J Dermatol. 2012;39(1):94-96. doi:10.1111/j.1346-8138.2011.01216.x
- Bienenfeld A, Nagler AR, Orlow SJ. Oral antibacterial therapy for acne vulgaris: An evidence-based review. Am J Clin Dermatol. 2017;18(4):469-490. doi:10.1007/s40257-017-0267-z
- Furustrand Tafin U, Corvec S, Betrisey B, et al. Role of rifampin against Propionibacterium acnes biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother. 2012;56(4):1885- 1891. doi:10.1128/AAC.05552-11
- Crawford WW, Crawford IP, Stoughton RB, et al. Laboratory induction and clinical occurrence of combined clindamycin and erythromycin resistance in Corynebacterium acnes. J Invest Dermatol. 1979;72(4):187-190. doi:10.1111/1523-1747.ep12676385
- Koyanagi S, Koizumi J, Nakase K, et al. Increased frequency of clindamycin-resistant Cutibacterium acnes strains isolated from Japanese patients with acne vulgaris caused by the prevalence of exogenous resistance genes. J Dermatol. 2023;50(6):793-799. doi:10.1111/1346-8138.16757
- Nakase K, Okamoto Y, Aoki S, et al. Long-term administration of oral macrolides for acne treatment increases macrolide-resistant Propionibacterium acnes. J Dermatol. 2018;45(3):340-343. doi:10.1111/1346-8138.14178
- Moon SH, Roh HS, Kim YH, et al. Antibiotic resistance of microbial strains isolated from Korean acne patients: Antibiotic resistance of microbial strains. J Dermatol. 2012;39(10):833-837. doi:10.1111/j.1346- 8138.2012.01626.x
- Ross JI, Eady EA, Cove JH, et al. 16S rRNA mutation associated with tetracycline resistance in a gram-positive bacterium. Antimicrob Agents Chemother. 1998;42(7):1702-1705. doi:10.1128/AAC.42.7.1702
- Cattoir V, Varca A, Greub G, et al. In vitro susceptibility of Actinobaculum schaalii to 12 antimicrobial agents and molecular analysis of fluoroquinolone resistance. J Antimicrob Chemother. 2010;65(12):2514-2517. doi:10.1093/jac/dkq383
- Ogawara H. Comparison of antibiotic resistance mechanisms in antibiotic-producing and pathogenic bacteria. Molecules. 2019;24(19):3430. doi:10.3390/molecules24193430
- Aslan Kayiran M, Karadag AS, Al-Khuzaei S, et al. Antibiotic resistance in acne: Mechanisms, complications and management. Am J Clin Dermatol. 2020;21(6):813-819. doi:10.1007/s40257-020-00556-6
- Sharma A, Gupta VK, Pathania R. Efflux pump inhibitors for bacterial pathogens: From bench to bedside. Indian J Med Res. 2019;149(2):129-145. doi:10.4103/ijmr.IJMR_2079_17
- Beirne C, McCann E, McDowell A, et al. Genetic determinants of antimicrobial resistance in three multi-drug resistant strains of Cutibacterium acnes isolated from patients with acne: a predictive in silico study. Access Microbiol. 2022;4(8):acmi000404. doi:10.1099/acmi.0.000404
- Aoki S, Nakase K, Nakaminami H, et al. Transferable multidrug-resistance Plasmid carrying a novel macrolide-clindamycin resistance gene, erm(50), in Cutibacterium acnes. Antimicrob Agents Chemother. 2020;64(3). doi:10.1128/AAC.01810-19
- Munita JM, Arias CA. Mechanisms of antibiotic resistance. Microbiol Spectr. 2016;4(2). doi:10.1128/microbiolspec. vmbf-0016-2015
- Ross JI, Eady EA, Carnegie E, et al. Detection of transposon Tn5432-mediated macrolide-lincosamide-streptogramin B (MLSB) resistance in cutaneous propionibacteria from six European cities. J Antimicrob Chemother. 2002;49(1):165-168. doi:10.1093/jac/49.1.165
- Koizumi J, Nakase K, Hayashi N, et al. Multidrug resistance Plasmid pTZC1 could be pooled among Cutibacterium strains on the skin surface. Microbiol Spectr. Published online 2023:e0362822. doi:10.1128/spectrum.03628-22
- Ross JI, Snelling AM, Carnegie E, et al. Antibiotic-resistant acne: lessons from Europe. Br J Dermatol. 2003;148(3):467-478. doi:10.1046/j.1365-2133.2003.05067.x
- Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16(3):e23-33. doi:10.1016/S1473-3099(15)00527-7
- Dumont-Wallon G, Moyse D, Blouin E, et al. Bacterial resistance in French acne patients. Int J Dermatol. 2010;49(3):283-288. doi:10.1111/j.1365-4632.2009.04270.x
- Nakase K, Nakaminami H, Takenaka Y, et al. Relationship between the severity of acne vulgaris and antimicrobial resistance of bacteria isolated from acne lesions in a hospital in Japan. J Med Microbiol. 2014;63(Pt 5):721-728. doi:10.1099/jmm.0.067611-0
- Sermswan P, Sriharat R, Saithong S, et al. A cross-sectional study examining the prevalence of antibiotic-resistant Cutibacterium acnes isolated from patients with acne in Bangkok, Thailand. J Dermatol. 2023;50(8):1008-1013. doi:10.1111/1346-8138.16823
- Luk NMT, Hui M, Lee HCS, et al. Antibiotic-resistant Propionibacterium acnes among acne patients in a regional skin centre in Hong Kong: Antibiotic-resistantP. acnein Hong Kong. J Eur Acad Dermatol Venereol. 2013;27(1):31- 36. doi:10.1111/j.1468-3083.2011.04351.x
- González R, Welsh O, Ocampo J, et al. Report: In vitro antimicrobial susceptibility of Propionibacterium acnes isolated from acne patients in northern Mexico: P. acnes resistance in Mexico. Int J Dermatol. 2010;49(9):1003- 1007. doi:10.1111/j.1365-4632.2010.04506.x
- Sardana K, Gupta T, Kumar B, et al. Cross-sectional pilot study of antibiotic resistance in Propionibacterium acnes strains in Indian acne patients using 16S-RNA polymerase chain reaction: A comparison among treatment modalities including antibiotics, benzoyl peroxide, and isotretinoin. Indian J Dermatol. 2016;61(1):45-52. doi:10.4103/0019-5154.174025
- Zhu T, Zhu W, Wang Q, et al. Antibiotic susceptibility of Propionibacterium acnes isolated from patients with acne in a public hospital in Southwest China: prospective cross-sectional study. BMJ Open. 2019;9(2):e022938. doi:10.1136/bmjopen-2018-022938
- Alkhawaja E, Hammadi S, Abdelmalek M, et al. Antibiotic resistant Cutibacterium acnes among acne patients in Jordan: a cross sectional study. BMC Dermatol. 2020;20(1):17. doi:10.1186/s12895-020-00108-9
- Zhang N, Yuan R, Xin KZ, et al. Antimicrobial susceptibility, biotypes and phylotypes of clinical Cutibacterium (formerly Propionibacterium) acnes strains isolated from acne patients: An observational study. Dermatol Ther (Heidelb). 2019;9(4):735-746. doi:10.1007/s13555-019-00320-7
- Nacht S, Yeung D, Beasley JN Jr, et al. Benzoyl peroxide: percutaneous penetration and metabolic disposition. J Am Acad Dermatol. 1981;4(1):31-37. doi:10.1016/s0190-9622(81)70004-5
- Leung AK, Barankin B, Lam JM, et al. Dermatology: how to manage acne vulgaris. Drugs Context. 2021;10:1-18. doi:10.7573/dic.2021-8-6
- Baldwin H, Elewski B, Hougeir F, et al. Sixty years of benzoyl peroxide use in dermatology. J Drugs Dermatol. 2023;22(1):54-59. doi:10.36849/JDD.7150
- Leyden JJ, Preston N, Osborn C, et al. In-vivo effectiveness of adapalene 0.1%/benzoyl peroxide 2.5% gel on antibiotic-sensitive and resistant Propionibacterium acnes. J Clin Aesthet Dermatol. 2011;4(5):22-26.
- Bandyopadhyay D. Topical antibacterials in dermatology. Indian J Dermatol. 2021;66(2):117-125. doi:10.4103/ijd. IJD_99_18
- Russell JJ. Topical therapy for acne. Am Fam Physician. 2000;61(2):357-366.
- Montes LF, Cordero AA, Kriner J, et al. Topical treatment of acne rosacea with benzoyl peroxide acetone gel. Cutis. 1983;32(2):185-190.
- Latter G, Grice JE, Mohammed Y, et al. Targeted topical delivery of retinoids in the management of acne vulgaris: Current formulations and novel delivery systems. Pharmaceutics. 2019;11(10):490. doi:10.3390/ pharmaceutics11100490
- Erlich M, Arie T, Koifman N, et al. Structure elucidation of silica-based core-shell microencapsulated drugs for topical applications by cryogenic scanning electron microscopy. J Colloid Interface Sci. 2020;579:778-785. doi:10.1016/j.jcis.2020.06.114
- Martin B, Meunier C, Montels D, et al. Chemical stability of adapalene and tretinoin when combined with benzoyl peroxide in presence and in absence of visible light and ultraviolet radiation. Br J Dermatol. 1998;139 Suppl 52:8- 11. doi:10.1046/j.1365-2133.1998.1390s2008.x
- Webster GF, Sugarman J, Levy-Hacham O, et al. Microencapsulated benzoyl peroxide and tretinoin for the treatment of acne vulgaris: Results from a phase 2 multicenter, double-blind, randomized, vehicle-controlled study. Skinmed. 2020;18(6):343-351.
- Leyden JJ, Hickman JG, Jarratt MT, et al. The efficacy and safety of a combination benzoyl peroxide/clindamycin topical gel compared with benzoyl peroxide alone and a benzoyl peroxide/erythromycin combination product. J Cutan Med Surg. 2001;5(1):37-42. doi:10.1177/120347540100500109
- Dermik Laboratories. BenzaClin Topical Gel [package insert]. US Food and Drug Administration. Accessed September 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/050756s040lbl.pdf
- Valeant Pharmaceuticals. Onexton [package insert]. US Food and Drug Administration. Revised November 2014. Accessed September 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/050819s012lbl.pdf
- Contract Pharmaceuticals Limited Niagra. Acanya [package insert]. US Food and Drug Administration. Revised October 2010. Accessed September 29, 2023.https://www.accessdata.fda.gov/drugsatfda_docs/ label/2010/050819s003lbl.pdf
- Sol-Gel Technologies. Twyneo [package insert]. US Food and Drug Administration. Revised July 2021. Accessed September 29, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214902s000lbl.pdf
- Valeant International. Retin-A [NDA]. US Food and Drug Administration. Accessed September 29, 2023. https:// www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=016921
- Stein Gold L, Baldwin H, Kircik LH, et al. Efficacy and safety of a fixed-dose clindamycin phosphate 1.2%, benzoyl peroxide 3.1%, and adapalene 0.15% gel for moderate-to-severe acne: A randomized phase II study of the first triple-combination drug. Am J Clin Dermatol. 2022;23(1):93-104. doi:10.1007/s40257-021-00650-3
- Gold LS, Kircik LH, Care PLLC PS, et al. 32970 Efficacy and safety of a fixed-dose clindamycin 1.2%, benzoyl peroxide 3.1%, and adapalene 0.15% gel for moderate-to-severe acne: Randomized phase 2 and phase 3 studies of the first triple-combination drug. J Am Acad Dermatol. 2022;87(3):AB50. doi:10.1016/j.jaad.2022.06.232
- Bausch Health Americas, Inc. Study to Compare the Safety and Efficacy of IDP-126 Gel to Epiduo® Forte Gel and Vehicle Gel Clinicaltrials.gov identifier: NCT04892706. Updated September 7, 2023. Accessed September 29, 2023. https://clinicaltrials.gov/study/NCT04892706
- Keshari S, Kumar M, Balasubramaniam A, et al. Prospects of acne vaccines targeting secreted virulence factors of Cutibacterium acnes. Expert Rev Vaccines. 2019;18(5):433-437. doi:10.1080/14760584.2019.1593830
- Wang Y, Hata TR, Tong YL, et al. The anti-inflammatory activities of Propionibacterium acnes CAMP factor-targeted acne vaccines. J Invest Dermatol. 2018;138(11):2355-2364. doi:10.1016/j.jid.2018.05.032
- Yu ACY, Volkers G, Jongkees SAK, et al. Crystal structure of the Propionibacterium acnes surface sialidase, a drug target for P. acnes-associated diseases. Glycobiology. 2022;32(2):162-170. doi:10.1093/glycob/cwab094
AUTHOR CORRESPONDENCE
Leon H. Kircik MD wedoderm@yahoo.com






