INTRODUCTION
Alopecia areata (AA) is a condition characterized by nonscarring hair loss. Cases of alopecia areata are most commonly seen in patients under age 30 and are frequently idiopathic. In this report, we discuss a woman in her 50s who developed AA shortly after receiving the Tdap vaccine and after one year of guselkumab therapy.
CASE
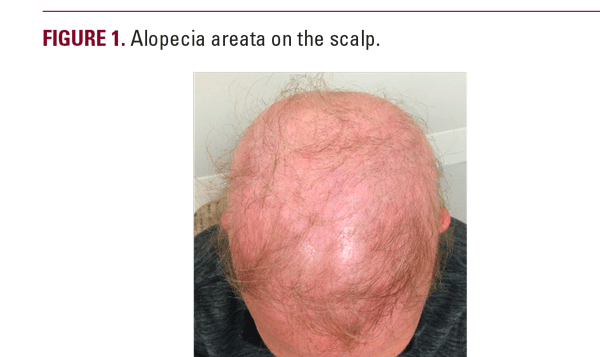
A woman in her 50s with history of psoriasis controlled with guselkumab for roughly two years presented to clinic for psoriasis follow-up. She had trialed numerous psoriasis therapeutics prior to guselkumab therapy including phototherapy, topical steroids, topical calcipotriene, and coal tar. She reported no side effects during her first 18 months of guselkumab therapy but, over the course of the next four months, she began to develop diffuse hair loss involving over 90% of her body including her scalp, eyebrows, eyelashes, and body hair, and was subsequently diagnosed with alopecia areata (Figure 1). Biopsy revealed alopecia areata with suppurative granulomatous inflammation. She reported her hair loss began shortly after receiving a Tdap booster vaccine. Given the potential implication of guselkumab as a causative agent for hair loss, she decided to discontinue guselkumab therapy. She was offered prednisone and methotrexate therapies for her psoriasis but declined both. She continued to follow up with dermatology regarding her psoriasis and alopecia areata.

DISCUSSION
The patient’s presentation of diffuse hair loss in the setting of a non-inflamed scalp is most consistent with alopecia areata (AA). While uncontrolled scalp psoriasis can lead to alopecia, this patient’s lack of active scalp psoriasis makes that diagnosis much less likely.
AA is a condition characterized by non-scarring hair loss that most commonly occurs in patients younger than 30 years.1 Although it is a genetically influenced autoimmune disease, its precise etiology is unknown. Onset after age 50 is rare, with one study revealing only 7.3% of AA patients within this age group.1
Guselkumab is a human monoclonal antibody that binds to the p19 subunit of IL-23, a key mediator of chronic inflammation and autoimmunity.2 While AA is not a typical side effect of guselkumab, AA has been reported as an adverse event of other biologic medications including ixekizumab,3 adalimumab,4 and ustekinumab.5-7 The underlying mechanism by which these medications could cause AA could include the activation of self-reactive T cells or a shift of T cells to Th1-predominant populations capable of destroying the hair follicle.3,5
The timing of AA onset in relation to this patient’s Tdap booster raises the question of whether her Tdap vaccination, and not the biologic treatment, contributed to her AA development. A review of Food and Drug Administration (FDA), Centers for Disease Control (CDC), and Vaccine Adverse Event Reporting System revealed 60 cases of hair loss following immunizations, the bulk of which occurred following hepatitis B vaccination.8 Additionally, alopecia universalis has been reported after tetanus vaccination, suggesting that the tetanus vaccine may be the cause of AA rather than the biologic treatment.9 The adjuvant property of vaccines offers a plausible mechanism by which vaccines could induce AA.
AA is a condition characterized by non-scarring hair loss that most commonly occurs in patients younger than 30 years.1 Although it is a genetically influenced autoimmune disease, its precise etiology is unknown. Onset after age 50 is rare, with one study revealing only 7.3% of AA patients within this age group.1
Guselkumab is a human monoclonal antibody that binds to the p19 subunit of IL-23, a key mediator of chronic inflammation and autoimmunity.2 While AA is not a typical side effect of guselkumab, AA has been reported as an adverse event of other biologic medications including ixekizumab,3 adalimumab,4 and ustekinumab.5-7 The underlying mechanism by which these medications could cause AA could include the activation of self-reactive T cells or a shift of T cells to Th1-predominant populations capable of destroying the hair follicle.3,5
The timing of AA onset in relation to this patient’s Tdap booster raises the question of whether her Tdap vaccination, and not the biologic treatment, contributed to her AA development. A review of Food and Drug Administration (FDA), Centers for Disease Control (CDC), and Vaccine Adverse Event Reporting System revealed 60 cases of hair loss following immunizations, the bulk of which occurred following hepatitis B vaccination.8 Additionally, alopecia universalis has been reported after tetanus vaccination, suggesting that the tetanus vaccine may be the cause of AA rather than the biologic treatment.9 The adjuvant property of vaccines offers a plausible mechanism by which vaccines could induce AA.
DISCLOSURES
Dr. Steven Feldman has received research, speaking, and/or consulting support from Galderma, GSK/Stiefel, Almirall, Alvotech, Leo Pharma, BMS, Boehringer Ingelheim, Mylan,






