Stay up-to-date on new clinical findings on Acne. View the latest articles, case reports, editorial features, supplements, Podcast episodes and more!
Stay up-to-date on new clinical findings on Acne. View the latest articles, case reports, editorial features, supplements, Podcast episodes and more!
Acne treatment can take weeks to deliver noticeable improvements, which may diminish patients’ perception of treatment effectiveness and undermine treatment adherence. Combination topical treatments that target multiple acne pathophysiological pathways are more efficacious than topical monotherapies, and simplifying combination treatment by delivering multiple active ingredients as fixed combinations may improve adherence.
Clindamycin phosphate 1.2%/adapalene 0.15%/benzoyl peroxide 3.1% gel (CAB) is the only fixed-dose triple-combination treatment approved for acne. This post hoc analysis assessed the impact of sex on efficacy and safety/tolerability of CAB.
Multiple treatment options exist for the management of moderate-to-severe acne. However, the comparative effectiveness (efficacy/safety) of moderate-to-severe acne treatments has not been systematically examined.
Although triple-combination therapies for acne are generally more efficacious than dual-combinations or topical monotherapy, this benefit may be offset by reduced adherence to a complicated treatment regimen. Clindamycin phosphate 1.2%/adapalene 0.15%/benzoyl peroxide 3.1% (CAB; Cabtreo®, Ortho Dermatologics) gel is the first triple-combination topical approved for the treatment of acne. By delivering multiple active ingredients as a fixed-dose combination, CAB gel may improve ease of use, which can benefit both treatment adherence and efficacy. The objective of this study was to compare the application characteristics of CAB gel with the layered application of its 3 individual active ingredients.
Topical tretinoin has been extensively studied in clinical trials, and its essential role in the treatment of acne vulgaris (acne) established through evidence-based guidelines. OBJECTIVE: To evaluate efficacy, safety, and tolerability of a novel tretinoin 0.05% lotion in moderate-to-severe acne in patients aged 9 years and older. Methods: A total of 1640 patients, 9-58 years of age were randomized to receive tretinoin 0.05% lotion or vehicle in two double-blind, placebo-controlled 12-week, 2-arm, parallel group studies evaluating safety and efficacy (inflammatory and noninflammatory lesion counts and acne severity using Evaluator Global Severity Scores [EGSS]). In addition, patients completed a patient satisfaction survey (PSS), Acne-specific quality of life (QoL) questionnaire and assessed their facial skin for shininess/oiliness improvement. The data from these two independent studies were pooled and analyzed.
Topical retinoids like tretinoin are a mainstay of acne treatment but associated cutaneous irritation and drying may lead to poor adherence. As vehicle optimization can improve patient preference and adherence, tretinoin 0.05% lotion (Altreno®) was formulated using polymeric emulsion technology for uniform delivery of micronized tretinoin and moisturizing/hydrating excipients. This study compared tolerability and participant preference of a branded tretinoin 0.05% lotion versus generic cream.
Oral isotretinoin remains a mainstay of treatment for severe, recalcitrant nodular acne. Novel formulations of isotretinoin have been developed over the past decade, including lidose isotretinoin and micronized isotretinoin. It is important to understand the differences between isotretinoin formulations to help guide clinical decision-making and selection of isotretinoin therapy. This study aims to provide evidence-based consensus statements regarding the use of novel formulations of isotretinoin for the treatment of moderate-to-severe acne. The Expert Consensus Group consisted of dermatologists with expertise in the treatment of acne. Voting members met in person to conduct a modified Delphi process; a maximum of 2 rounds of voting were conducted for each consensus statement. A total of 5 statements were generated regarding the use of novel formulations of isotretinoin, addressing the efficacy, tolerability, and side effects of novel isotretinoin formulations. All 5 statements achieved agreement with high consensus. The Expert Consensus Group agrees that individualized selection of isotretinoin therapy is important to maximize efficacy and minimize side effects. Compared to generic isotretinoin, micronized isotretinoin may require lower doses to achieve sufficient plasma concentrations. With the increased bioavailability of micronized formulation, there is no need to calculate cumulative dose; instead, the general recommendation with micronized isotretinoin is to treat for at least 5 months, or longer if needed to achieve clearance. Micronized isotretinoin can be taken in the fed or fasted state and has an acceptable safety profile.
A once-daily, three-pronged approach using an antibiotic, antibacterial, and retinoid may provide faster acne improvement versus monotherapy or dual-combination products. This post hoc analysis compared threshold acne lesion reductions with clindamycin phosphate 1.2%/adapalene 0.15%/benzoyl peroxide 3.1% (CAB) gel — the first FDA-approved triple-combination topical acne product — to its dyads and vehicle.
Topical tretinoin has historically been limited by poor tolerability and molecular instability. Research advances have enhanced its efficacy and tolerability, along with reducing oxidation and photodegradation. By overcoming historical limitations, tretinoin use can be extended to patient populations and clinical situations previously not suitable. This review discusses historical limitations of tretinoin, methods employed to overcome those limitations, use within clinical practice, and new formulations of tretinoin for the treatment of acne.
Tazarotene has been extensively studied in clinical trials and is widely used to treat acne vulgaris (acne), with data suggesting that is one of the most potent topical retinoids. Irritation from the cream, foam, and gel formulations has limited its use in clinical practice.
Isotretinoin is considered the gold standard treatment for severe nodulocystic acne, though it has been the subject of controversy in the media for concerns related to adverse psychiatric effects. In 2005, the FDA issued a black box warning for suicide, depression, aggression, and psychosis.1
Acne scarring can have a detrimental impact on quality of life, making early and effective treatment essential. Lasers and energy-based treatment are the preferred treatment modality, yet a clear consensus on the ideal laser selection is lacking in the literature.
The use of alpha-hydroxy acids (AHAs) and beta-hydroxy acids (BHAs) for acne treatment and their integration into daily skincare routines for better cosmetic outcomes has increasingly become a norm, particularly in light of steep insurance deductibles and restricted access to prescription options. This article presents an analysis from a single-center, open-label study with 20 participants conducted to evaluate if an over-the-counter (OTC) barrier-restoring cream gel can alleviate the adverse skin reactions caused by a conventional dermatologist-recommended acne treatment plan in individuals with mild to moderate acne over 6 weeks. This evaluation encompassed both objective clinical assessments of facial skin health and subjective reports of product tolerability, complemented by before-and-after images and questionnaires to capture consumer feedback on the OTC moisturizer as well as an OTC salicylic acid-containing cleanser and spot treatment.

One of the key characteristics of acne is post-inflammatory hyperpigmentation and is most noticeable in darker skin tones. It is also important to point out that keloidal or hypertrophic scarring may result and should be considered when treating patients.
Here we can appreciate the erythema of inflammatory papules in lighter skin tones and the brown coloration of inflammatory papules mimicking post-inflammatory hyperpigmentation in darker skin tones.
Do not pop, rather pick a therapeutic strategy from the wide array of new….oh wait, nevermind. The world of acne treatment R&D is unfortunately shrinking as payors pick away at the medical necessity of managing the most common inflammatory skin disease. Like Marty McFly fading in that famous family photo, the spotlight on acne is shifting, enough to make Dr. Pimple Punisher Dr. Hilary Baldwin pick up a bottle of Prozac. But all hope is not lost.
Join JDD Podcast host Dr. Adam Friedman as he and Dr. Baldwin scour the therapeutic landscape and take a sharp left into the over the counter space to review accessible options to manage both the today and tomorrow of Acne Vulgaris, the symbiosis between RX and OTC to address barrier dysfunction, scarring and hyperpigmentation.
 Exploring the Potential Link Between Minoxidil Use and Rosacea Using A Real-World Data Base
Exploring the Potential Link Between Minoxidil Use and Rosacea Using A Real-World Data Base
 GLP-1 Receptor Agonists in Overweight and Obese Patients With Hidradenitis Suppurativa
GLP-1 Receptor Agonists in Overweight and Obese Patients With Hidradenitis Suppurativa
 Non-Invasive Diagnosis of Sun Damaged Skin: Actinic Keratosis Vs Squamous Cell Carcinoma
Non-Invasive Diagnosis of Sun Damaged Skin: Actinic Keratosis Vs Squamous Cell Carcinoma
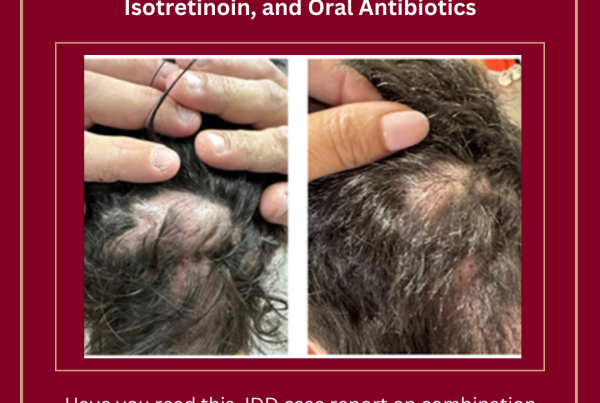 Treatment Strategy for Refractory Dissecting Cellulitis of the Scalp Using Bimekizumab, Isotretinoin, and Oral Antibiotics
Treatment Strategy for Refractory Dissecting Cellulitis of the Scalp Using Bimekizumab, Isotretinoin, and Oral Antibiotics