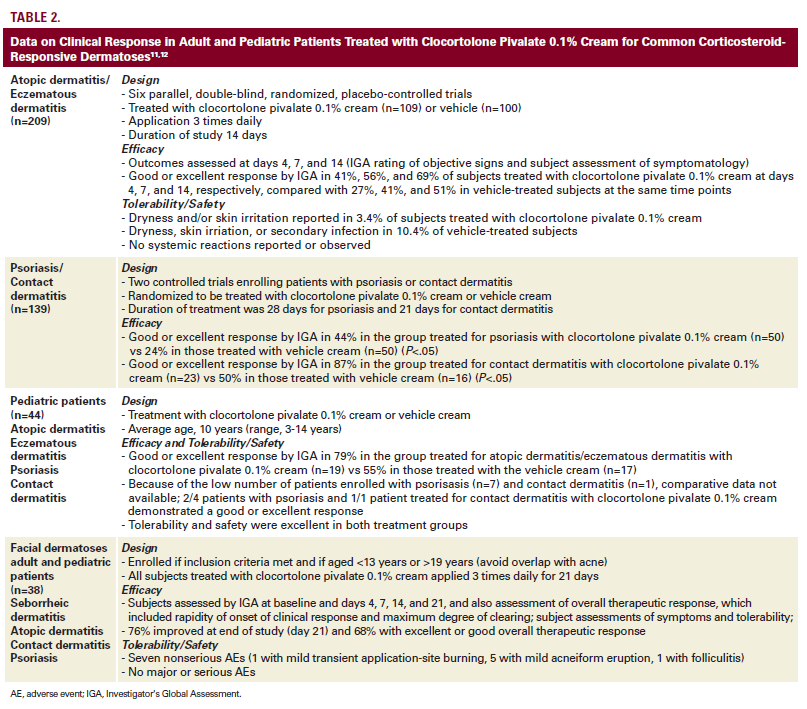
tively treated with clocortolone pivalate 0.1% cream.11,12 Overall,
the efficacy and safety outcomes observed in these clinical trials
were favorable as depicted in Table 2.
Treatment of Facial Dermatoses
In clinical trials with clocortolone pivalate 0.1% cream, facial application
over the designated study durations (range 2-4 weeks)
was included for 147 study patients, especially those affected
by SD and AD.11 In this subset, the efficacy, tolerability, and
safety outcomes were consistent with the overall results from
the trial. The results of an analysis of patients with facial dermatoses
who were treated with clocortolone pivalate 0.1% cream
3 times daily for 21 days are shown in Table 2. It is important
that use of any TCS on the face be limited in duration through
proper monitoring, with treatment-free periods interspersed
as necessary if chronic administration is required for disease
control. In addition, avoidance of prolonged continuous application
to the eyelids and intertriginous areas is advised as a
general caution with TCS use.
Additional Data of Clinical Relevance
A subset of study subjects with chronic eczematous dermatoses
and psoriasis (n=27) were treated over more prolonged durations
with clocortolone pivalate 0.1% cream at sites of active
skin disease (mean 116.4 days).11 No adverse reactions related
to treatment were noted other than mild dryness in one patient.
The use of any TCS to adequately manage a given skin disorder
over prolonged durations of therapy warrants appropriate






