tolerability of application of the study product, and the frequency and duration of adverse events.AssessmentsInvestigator AssessmentsThroughout the study, efficacy assessments were performed on the treatment areas for each subject by the same Live Treating Investigator at baseline, weeks 4 and 8. The efficacy parameters were Validated Assessment Scales for facial wrinkles (Fitzpatrick Wrinkle Scale), Global Aesthetic Improvement Scale (GAIS) and a customized 10-point scale for the assessment of skin quality, Visual Assessment of Skin (VAS) Quality and Photodamage (Figure 1).SafetyLocal (dermal) tolerability examination (of the face and neck separately) was performed at all study visits and will include assessments of stinging/burning (rated by the patient verbally), dryness, scaling, edema, and erythema (rated by the investigator or appropriately trained designee). Local tolerability on the face and neck was rated as none, mild, moderate, or severe.Standardized PhotographyStandardized photographs were taken at 0°, 45°, and 315° angles using the Visia® CR system (Canfield Imaging Systems, Fairfield). For each angle, a set of four different photographs will be taken including non-polarized, cross-polarized and parallel-polarized white light as well as UV light images.Statistical AnalysisOrdinal variables were analyzed using Wilcoxon test for paired samples. For ratio scaled variables normal distribution was verified by Kolmogorov-Smirnoff test and then analyzed using Student-T test for paired variables or Wilcoxon test. All statistical tests were two-sided and tested in conjunction with a 0.05 nominal significance level. Analyses were carried out using SAS version 9.4 statistical software (Cary, NC).
RESULTS
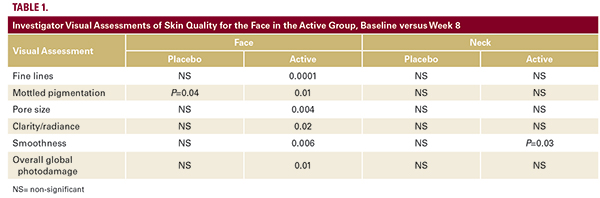
Population20 subjects total (2 male and 18 female) with an average age of 54.9 (±7.5) years were enrolled into this study. Two female subjects (1 active and 1 placebo) were lost to follow- up prior to the completion of the study and were not included in the statistical analysis of the results. All female subjects were peri (1) or post-menopausal (17). All but one subject were non-smokers, and all occasionally use alcohol. Patients had skin type I (n=1), skin type II (n=8), skin type III (n=6), and skin type IV (n=5).Investigator AssessmentsNo statistically significant differences were found in baseline ratings of investigator visual assessments of skin quality for the face between active and placebo groups for all assessment parameters (fine lines, mottled pigmentation, pore size, clarity/radiance, laxity, and overall global photodamage). Between baseline and week 8, there was an improvement from mild to moderate in Fitzpatrick Wrinkle Scale scores the active group, for face and neck, but not the placebo group. The results however did not reach statistical significance. Statistically significant improvements were noted in the investigator visual assessments of skin quality for the face in the active group, baseline versus week 8, in fine lines, mottled pigmentation, pore size, clarity/radiance, smoothness, and overall global photodamage (Table 1, Figure 2, 3). In the placebo group a statistically significant improvement was noted in mottled pigmentation for facial skin, baseline vs. week 8 (P=0.04). No other factors displayed significant change. When evaluating the neck, statistically significant improvement was noted in the investigator visual assessments of skin quality in the active group, baseline versus week 8, in smoothness (P=0.03). No other factors displayed significant change, and no statistically significant results were noted in the placebo group for neck skin, baseline vs week 8, for investigator visual assessments. Assessments of the face, but not the neck, by both the treating and blinded investigator, demon