
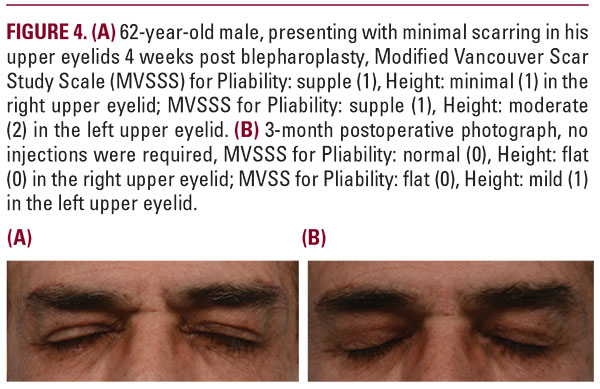
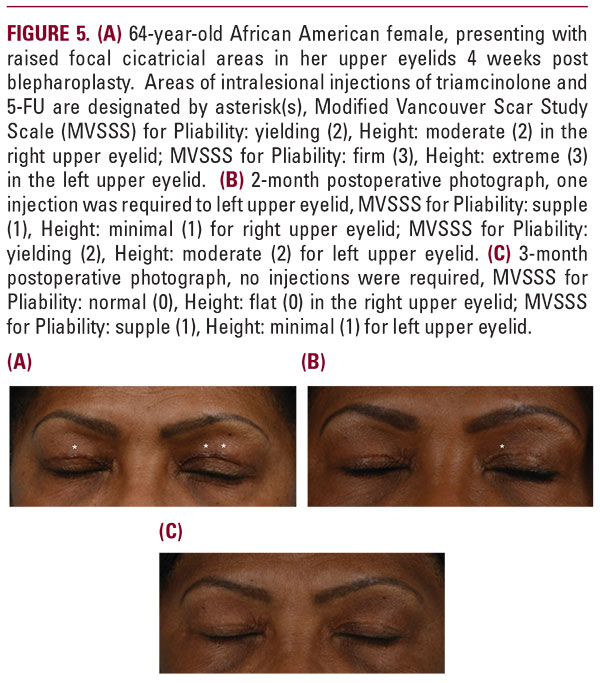
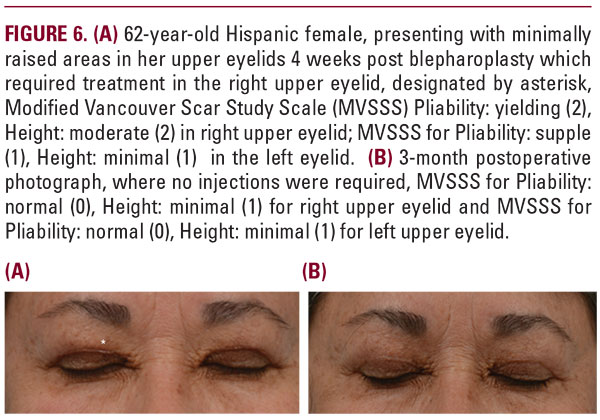
 More specifically, a total of 113 intralesional injections were performed over the course of 6 months follow-up in both groups. Among the injection treatments performed, 73.5% (83 treatments) were performed at 1 month, 17.7% (20 treatments) at 2 months, 4.4% (5 treatments) at 3 months, 3.5% (4 treatments) at 5 months, and 0.9% (1 treatment) at 6 months. A similar distribution of injection treatments was observed between the two groups with the majority occurring 4 weeks after undergoing blepharoplasty; see Figure 2.There were no adverse reactions reported from either group, nor were there any reported incidence of post injection thinningof skin, pigmentary changes, nor irritation. All patients tolerated SKN2017B well. All patients were able to reach an end point of Grade 1 or better in the Pliability and Height parameters of the Vancouver Scar Study Scale at the conclusion of their treatment. No patient required post procedure dermabrasion, laser resurfacing, or surgical revision.
More specifically, a total of 113 intralesional injections were performed over the course of 6 months follow-up in both groups. Among the injection treatments performed, 73.5% (83 treatments) were performed at 1 month, 17.7% (20 treatments) at 2 months, 4.4% (5 treatments) at 3 months, 3.5% (4 treatments) at 5 months, and 0.9% (1 treatment) at 6 months. A similar distribution of injection treatments was observed between the two groups with the majority occurring 4 weeks after undergoing blepharoplasty; see Figure 2.There were no adverse reactions reported from either group, nor were there any reported incidence of post injection thinningof skin, pigmentary changes, nor irritation. All patients tolerated SKN2017B well. All patients were able to reach an end point of Grade 1 or better in the Pliability and Height parameters of the Vancouver Scar Study Scale at the conclusion of their treatment. No patient required post procedure dermabrasion, laser resurfacing, or surgical revision.DISCUSSION
Undesirable scarring following a surgical procedure can result in emotional distress for the patient, as well as the surgeon. Therefore, it is of great importance to help prevent abnormal scarring, and appropriately manage scars to improve patient






