 temperatures above 45 C to be uncomfortably hot. After the initial heat build phase, treatment fluence is adjusted to the point of discomfort in the range of 3-4/10 on a pain scale. This range-finding places the tissue temperature in the therapeutic range. A patient reporting a discomfort score of 5 out of 10 may experience pain or overheating. A patient who reports a discomfort score of 1 or 2 may not realize the maximum potential results. Thus, it is no accident that the treatment was not reported as painful by the patients since the fluences were tailored to achieve that result. The contour reductions presented here were produced at these comfortable fluences.The contour reductions showed no sharp transition at the edge areas of the treatment. Given the application method, applicators attached via a belt and frame system, both light scattering and heat feathering are likely at play here. Tapering of the temperature gradient and therefore the fat reduction effect at or beyond the edge of the applicator would be expected and matches clinical observations in this study.The use of a light-based and heat-based method raises several concerns and possibilities. The potential for thermal effects from melanin absorption in the skin are potentially concerning. Multiple Fitzpatrick VI patients were treated with no blisters or other skin effects observed. The thermodynamic problem of competing contact cooling of the skin and deeper heating of subcutaneous fat was solved in this experimental device producing fat layer reduction without skin adverse events. Heat produces various effects that cold exposure may not produce or produce in equal measure including release of heat shock proteins, among other effects. The potential to allow some heating of the skin with a small modification of parameters exists. Even with the existing exposure, fibrous septae running between the skin and fascia are within the heated zone and likely to show some response to the hyperthermic exposure during healing.Exposure time in this study was 25 minutes. This allows treatment in significantly less time than antecedent devices. Sincemany patients desire to treat more than a single small bulge, the time savings, multiplied over several or many treatment cycles, becomes substantial. One of the principal patient motivations in selecting a non-surgical option is ease, convenience and no need to disrupt the normal life schedule. Time savings is a premium issue in this context that cannot be overstated.
temperatures above 45 C to be uncomfortably hot. After the initial heat build phase, treatment fluence is adjusted to the point of discomfort in the range of 3-4/10 on a pain scale. This range-finding places the tissue temperature in the therapeutic range. A patient reporting a discomfort score of 5 out of 10 may experience pain or overheating. A patient who reports a discomfort score of 1 or 2 may not realize the maximum potential results. Thus, it is no accident that the treatment was not reported as painful by the patients since the fluences were tailored to achieve that result. The contour reductions presented here were produced at these comfortable fluences.The contour reductions showed no sharp transition at the edge areas of the treatment. Given the application method, applicators attached via a belt and frame system, both light scattering and heat feathering are likely at play here. Tapering of the temperature gradient and therefore the fat reduction effect at or beyond the edge of the applicator would be expected and matches clinical observations in this study.The use of a light-based and heat-based method raises several concerns and possibilities. The potential for thermal effects from melanin absorption in the skin are potentially concerning. Multiple Fitzpatrick VI patients were treated with no blisters or other skin effects observed. The thermodynamic problem of competing contact cooling of the skin and deeper heating of subcutaneous fat was solved in this experimental device producing fat layer reduction without skin adverse events. Heat produces various effects that cold exposure may not produce or produce in equal measure including release of heat shock proteins, among other effects. The potential to allow some heating of the skin with a small modification of parameters exists. Even with the existing exposure, fibrous septae running between the skin and fascia are within the heated zone and likely to show some response to the hyperthermic exposure during healing.Exposure time in this study was 25 minutes. This allows treatment in significantly less time than antecedent devices. Sincemany patients desire to treat more than a single small bulge, the time savings, multiplied over several or many treatment cycles, becomes substantial. One of the principal patient motivations in selecting a non-surgical option is ease, convenience and no need to disrupt the normal life schedule. Time savings is a premium issue in this context that cannot be overstated.CONCLUSION
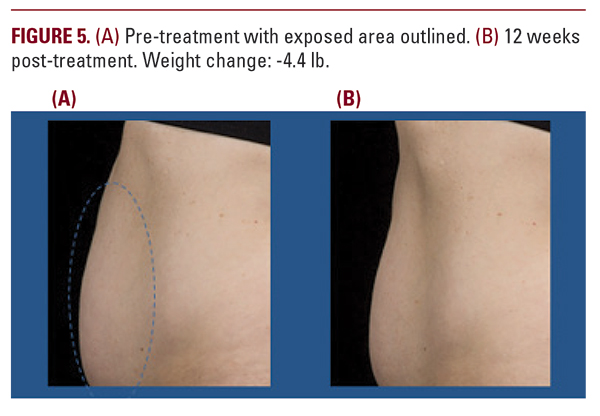
The current study is a prospective, controlled (treatment versus no-treatment) study to evaluate a non-invasive 1060 nm diode laser with contact cooling intended for non-invasive treatment of the abdomen to achieve disruption and removal of adipocytes for contour reduction. In conjunction with the study of flank treatment with the same device (Katz 2015), this formed the pivotal study data for FDA approval that has since been granted.The data showed the 1060 nm laser hyperthermic treatment produced a consistent, observable reduction in abdominal contour compared to baseline based on ultrasound measurements and grading by blinded evaluators. Subjects are highly satisfied with the treatment, which is safe with only mild and transient side effects. No serious adverse events or unanticipated adverse device events were encountered. Treatment time is reduced compared to currently available alternative technologies with an easily tolerable treatment discomfort profile comparable to alternatives and minimal if any recovery discomfort.
DISCLOSURES
Both authors served as consultants for the study sponsor as well as serving on the speakers bureau. Dr. Doherty serves as medical director for the study sponsor.
ACKNOWLEDGMENTS
Presented in part at the American Society for Laser Medicine & Surgery Annual Meeting, April 2015, Orlando, FL. This study was funded by Cynosure Inc., Westford, MA as part of a Phase III FDA clinical study.






